Credentialing Guidelines and Requirements a Candidate Guidebook Table of Contents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Betamethasone
Betamethasone Background Betamethasone is a potent, long-acting, synthetic glucocorticoid widely used in equine veterinary medicine as a steroidal anti-inflammatory.1 It is often administered intra-articularly for control of pain associated with inflammation and osteoarthritis.2 Betamethasone is a prescription medication and can only be dispensed from or upon the request of a http://en.wikipedia.org/wiki/Betamethasone#/media/File:Betamethasone veterinarian. It is commercially available .png in a variety of formulations including BetaVet™, BetaVet Soluspan Suspension® and Betasone Aqueous Suspension™.3 Betamethasone can be used intra-articularly, intramuscularly, by inhalation, and topically.4 When administered intra-articularly, it is often combined with other substances such as hyaluronan.5 Intra-articular and intramuscular dosages range widely based upon articular space, medication combination protocol, and practitioner preference. Betamethasone is a glucocorticoid receptor agonist which binds to various glucocorticoid receptors setting off a sequence of events affecting gene transcription and the synthesis of proteins. These mechanisms of action include: • Potential alteration of the G protein-coupled receptors to interfere with intracellular signal transduction pathways • Enhanced transcription in many genes, especially those involving suppression of inflammation. • Inhibition of gene transcription – including those that encode pro-inflammatory substances. The last two of these are considered genomic effects. This type of corticosteroid effect usually occurs within hours to days after administration. The genomic effects persist after the concentrations of the synthetic corticosteroid in plasma are no longer detectable, as evidenced by persistent suppression of the normal production of hydrocortisone following synthetic corticosteroid administration.6 When used judiciously, corticosteroids can be beneficial to the horse. -

Surfen, a Small Molecule Antagonist of Heparan Sulfate
Surfen, a small molecule antagonist of heparan sulfate Manuela Schuksz*†, Mark M. Fuster‡, Jillian R. Brown§, Brett E. Crawford§, David P. Ditto¶, Roger Lawrence*, Charles A. Glass§, Lianchun Wang*, Yitzhak Torʈ, and Jeffrey D. Esko*,** *Department of Cellular and Molecular Medicine, Glycobiology Research and Training Center, †Biomedical Sciences Graduate Program, ‡Department of Medicine, Division of Pulmonary and Critical Care Medicine and Veteran’s Administration San Diego Medical Center, ¶Moores Cancer Center, and ʈDepartment of Chemistry and Biochemistry, University of California at San Diego, La Jolla, CA 92093; and §Zacharon Pharmaceuticals, Inc, 505 Coast Blvd, South, La Jolla, CA 92037 Communicated by Carolyn R. Bertozzi, University of California, Berkeley, CA, June 18, 2008 (received for review May 26, 2007) In a search for small molecule antagonists of heparan sulfate, Surfen (bis-2-methyl-4-amino-quinolyl-6-carbamide) was first we examined the activity of bis-2-methyl-4-amino-quinolyl-6- described in 1938 as an excipient for the production of depot carbamide, also known as surfen. Fluorescence-based titrations insulin (16). Subsequent studies have shown that surfen can indicated that surfen bound to glycosaminoglycans, and the extent block C5a receptor binding (17) and lethal factor (LF) produced of binding increased according to charge density in the order by anthrax (18). It was also reported to have modest heparin- heparin > dermatan sulfate > heparan sulfate > chondroitin neutralizing effects in an oral feeding experiments in rats (19), sulfate. All charged groups in heparin (N-sulfates, O-sulfates, and but to our knowledge, no further studies involving heparin have carboxyl groups) contributed to binding, consistent with the idea been conducted, and its effects on HS are completely unknown. -

268 Part 522—Implantation Or Injectable Dosage Form
§ 520.2645 21 CFR Ch. I (4–1–18 Edition) (ii) Indications for use. For the control 522.82 Aminopropazine. of American foulbrood (Paenibacillus 522.84 Beta-aminopropionitrile. larvae). 522.88 Amoxicillin. 522.90 Ampicillin injectable dosage forms. (iii) Limitations. The drug should be 522.90a Ampicillin trihydrate suspension. fed early in the spring or fall and con- 522.90b Ampicillin trihydrate powder for in- sumed by the bees before the main jection. honey flow begins, to avoid contamina- 522.90c Ampicillin sodium. tion of production honey. Complete 522.144 Arsenamide. treatments at least 4 weeks before 522.147 Atipamezole. main honey flow. 522.150 Azaperone. 522.161 Betamethasone. [40 FR 13838, Mar. 27, 1975, as amended at 50 522.163 Betamethasone dipropionate and FR 49841, Dec. 5, 1985; 59 FR 14365, Mar. 28, betamethasone sodium phosphate aque- 1994; 62 FR 39443, July 23, 1997; 68 FR 24879, ous suspension. May 9, 2003; 70 FR 69439, Nov. 16, 2005; 73 FR 522.167 Betamethasone sodium phosphate 76946, Dec. 18, 2008; 75 FR 76259, Dec. 8, 2010; and betamethasone acetate. 76 FR 59024, Sept. 23, 2011; 77 FR 29217, May 522.204 Boldenone. 17, 2012; 79 FR 37620, July 2, 2014; 79 FR 53136, 522.224 Bupivacaine. Sept. 8, 2014; 79 FR 64116, Oct. 28, 2014; 80 FR 522.230 Buprenorphine. 34278, June 16, 2015; 81 FR 48702, July 26, 2016] 522.234 Butamisole. 522.246 Butorphanol. § 520.2645 Tylvalosin. 522.275 N-Butylscopolammonium. 522.300 Carfentanil. (a) Specifications. Granules containing 522.304 Carprofen. 62.5 percent tylvalosin (w/w) as 522.311 Cefovecin. -

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredients Catalog HPD-5E ® CREATING A HEALTHY WORLDTM Active Pharmaceutical Ingredients (APIs) Available for International Markets Human Pharmaceutical Department www.Pharmapex.net Catalog HPD-5E *Not all products referred to on this site are available in all countries and our products are subject to different regulatory requirements depending on the country of use. Consequently, certain sections of this site may be indicated as being intended only for users in specic countries. Some of the products may also be marketed under different trade names. You should not construe anything on this site as a promotion or solicitation for any product or for the use of any product that is not authorized by the laws and regulations of your country of residence. For inquiries about the availability of any specic product in your country, you may simply contact us at [email protected]. **Products currently covered by valid US Patents may be offered for R&D use in accordance with 35 USC 271(e)+A13(1). Any patent infringement and resulting liability is solely at buyer risk. ©2016, Pharmapex USA, A member of Apex Group of Companies, All Rights Reserved. Toll-Free: 1.844.PHARMAPEX Fax: + 1.619.881.0035 ACTIVE PHARMACEUTICAL [email protected] CREATING A HEALTHY WORLD™ www.Pharmapex.net INGREDIENTS About Pharmapex’s Human Pharmaceuticals Department: Pharmapex’s Human Pharmaceuticals Department (HPD) is a leading source for high-quality Active Pharmaceutical Ingredients (APIs) and Finished Pharmaceutical Products (FPPs) in various markets across the globe. With an extensive product portfolio, our consortium of companies is dedicated to addressing and solving the most important medical needs of our time, including oncology (e.g., multiple myeloma and prostate cancer), neuroscience (e.g., schizophrenia, dementia and pain), infectious disease (e.g., HIV/AIDS, Hepatitis C and tuberculosis), and cardiovascular and metabolic diseases (e.g., diabetes). -

Product Portfolio
API Product Portfolio Hovione has developed a range of particle engineered Particle Engineered APIs APIs with superior powder properties that will address the challenges of your formulation development. Corticosteroids PRODUCT ROUTE OF CAS CUSTOMIZED REGULATORY ADMINISTRATION NUMBER PARTICLE SIZE STATUS Beclomethasone Dipropionate Anhydrous Inhalation Nasal Oral Topical 5534-09-08 Beclomethasone Dipropionate Monohydrate Inhalation Nasal 77011-63-3 US DMF Betamethasone Acetate Ophtalmic Injectable 987-24-6 US DMF Betamethasone Disodium Phosphate Ophtalmic Injectable Topical 151-73-5 US DMF, JP DMF Fluticasone Furoate Inhalation Nasal 397864-44-7 US DMF CEP, US DMF, JP DMF, Fluticasone Propionate Inhalation Nasal Topical 80474-14-2 CN DMF Halobetasol Propionate Topical 66852-54-8 US DMF Hydrocortisone Aceponate Topical 74050-20-7 ASMF & VMF Mometasone Furoate Anhydrous Inhalation Nasal Topical 83919-23-7 CEP, US DMF, JP DMF Mometasone Furoate Monohydrate Inhalation Nasal 141646-00-6 CEP, US DMF, JP DMF LABA/LAMA PRODUCT ROUTE OF CAS CUSTOMIZED REGULATORY ADMINISTRATION NUMBER PARTICLE SIZE STATUS Aclidinium Bromide Inhalation 320345-99-1 US DMF Glycopyrronium Bromide Inhalation 51186-83-5 US DMF Salmeterol Xinafoate Inhalation 94749-08-3 CEP, US DMF Indacaterol Maleate Inhalation 312753-06-3 DEV Olodaterol Sulphate Inhalation 869477-96-3 DEV Salbutamol Inhalation 18559-94-9 GMP, TECH PACK Tiotropium Bromide Monohydrate Inhalation 136310-93-5 DEV Umeclidinium Bromide Inhalation 869113-09-7 DEV, TECH PACK Vilanterol Trifenatate Inhalation 503070-58-4 GMP, TECH PACK Hovione provides the peace of mind of a high quality Security of supply manufacturer who is a founding member of Rx-360 and has an unblemished regulatory track record. -

Product List
Arena Product List A Cabazitaxel (Under Development) Acebutolol HCl Calcitriol N-Acetyl-L-Asparagine Calcifediol N-Acetyl-L-Aspartic Acid Calcipotriol Monohydrate Acexamic Acid (Upon Request) Canrenoate K Aesculin Canrenone micronised Agomelatine (Under Development) Capecitabine Alfacalcidol Captopril Alimemazine Tartrate Carbamizole Aliskiren Fumarate (Under Development) Carbasalate Calcium Altizide Carbomers Amcinonide Acetyl Thiazolidin Carboxylic Acid Amifostine L-Carnitine Base Amiodarone HCl (Upon Request) Acetyl-L-Carnitine HCl Amisulpride (Upon Request) Acetyl Carnosine Amitriptyline HCl L-Carnosine Apomorphine HCl Cefaclor L-Arginine Base Cefalexin Monohydrate L-Arginine-L-Aspartate Cefamandole Nafate Sterile L-Arginine-L-Glutamate Cefazedone Sodium Sterile Aripiprazole (Under Development) Cefepime HCL Artesunate (Upon Request) Cefmetazole Artemether (Upon Request) Cefmetazole Sodium Sterile Articaine HCl Cefminox Sodium Sterile L-Asparagine Anyhdrous Cefodizime Disodium L-Asparagine Monohydrate Cefodizime Sodium Sterile DL-Aspartic Acid Magnesium Salt Cefotetan DL-Aspartic Acid Magnesium + Potassium Salt Cefotetan Sodium Sterile DL-Aspartic Acid Potassium Salt Cefozopran (Under Development) L-Aspartic Acid Sodium Salt Monohydrate Cefsulodin (Under Development) Atovaquone (Under Development) Cefsulodin Sodium Sterile Azacitidine (Under Development) Cefuroxime Sodium Azathioprine Powder Cefuroxime Sodium Sterile Centella Asiatica Extract B Cephacetrile Sodium Sterile Baclofen Cetirizine HCl Beclomethasone Base Cetrimide Powder -

Kentucky Horse Racing Commission Withdrawal Guidelines Thoroughbred; Standardbred; Quarter Horse, Appaloosa, and Arabian KHRC 8-020-2 (11/2018)
Kentucky Horse Racing Commission Withdrawal Guidelines Thoroughbred; Standardbred; Quarter Horse, Appaloosa, and Arabian KHRC 8-020-2 (11/2018) General Notice Unless otherwise specified in these withdrawal guidelines or the applicable regulations and statutes, the following withdrawal guidelines are voluntary and advisory. The guidelines are recommendations based on current scientific knowledge that may change over time. A licensee may present evidence of full compliance with these guidelines to the Kentucky Horse Racing Commission (the “Commission” or “KHRC”) and the stewards as a mitigating factor to be used in determining violations and penalties. These withdrawal interval guidelines assume that administration of medications will be performed at doses that are not greater than the manufacturer’s maximum recommended dosage. Medications administered at dosages above manufacturer’s recommendations, in compounded formulations and/or in combination with other medications and/or administration inside the withdrawal interval may result in test sample concentrations above threshold concentrations that could lead to positive test results and the imposition of penalties. The time of administration of an orally administered substance, for the purposes of withdrawal interval, shall be considered to be the time of complete ingestion of the medication by the horse via eating or drinking. Brand names of medications, where applicable, are listed in parentheses or brackets following the generic name of a drug. In addition to the requirements contained in KRS Chapter 13A, the KHRC shall give notice of an amendment or addition to these withdrawal guidelines by posting the change on the KHRC website and at all Kentucky racetracks at least two weeks before the amendment or addition takes legal effect. -
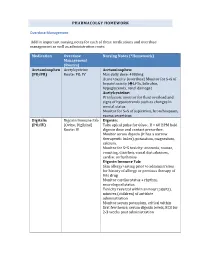
PHARMACOLGY HOMEWORK Overdose Management Add In
PHARMACOLGY HOMEWORK Overdose Management Add in important nursing notes for each of these medications and overdose management as well as administration route. Medication Overdose Nursing Notes (*Homework) Management (Routes) Acetaminophen Acetylcysteine Acetaminophen: (PO/PR) Route: PO, IV Max daily dose: 4000mg Acute toxicity (overdose) Monitor for S+S of hepatotoxicity (éLFTs, bilirubin, hypoglycemia, renal damage) Acetylcysteine: IV infusion: monitor for fluid overload and signs of hyponatremia such as changes in mental status Monitor for S+S of aspiration, bronchospasm, excess secretions Digitalis Digoxin Immune Fab Digoxin: (PO/IV) (Ovine, Digibind) Take apical pulse for 60sec. If < 60 BPM hold Route: IV digoxin dose and contact prescriber. Monitor serum digoxin (it has a narrow therapeutic index), potassium, magnesium, calcium. Monitor for S+S toxicity: anorexia, nausea, vomiting, diarrhea, visual disturbances, cardiac arrhythmias Digoxin Immune Fab: Skin allergy testing prior to administration for history of allergy or previous therapy of this drug Monitor cardiac status + rhythm, neurological status Toxicity reversal within an hour (adults), minutes (children) of antidote administration Monitor serum potassium, critical within first few hours; serum digoxin levels, ECG for 2-3 weeks post administration Heparin Protamine sulfate Heparin: (IV/SC) (IV) Monitor for spontaneous bleeding, thrombocytopenia Monitor aPTT levels Protamine Sulfate: Sudden drop in BP Monitor BP+ P q15-30 min Monitor aPTT Opioid Naloxone (IV-adults, Opioids: -

Jp Xvii the Japanese Pharmacopoeia
JP XVII THE JAPANESE PHARMACOPOEIA SEVENTEENTH EDITION Official from April 1, 2016 English Version THE MINISTRY OF HEALTH, LABOUR AND WELFARE Notice: This English Version of the Japanese Pharmacopoeia is published for the convenience of users unfamiliar with the Japanese language. When and if any discrepancy arises between the Japanese original and its English translation, the former is authentic. The Ministry of Health, Labour and Welfare Ministerial Notification No. 64 Pursuant to Paragraph 1, Article 41 of the Law on Securing Quality, Efficacy and Safety of Products including Pharmaceuticals and Medical Devices (Law No. 145, 1960), the Japanese Pharmacopoeia (Ministerial Notification No. 65, 2011), which has been established as follows*, shall be applied on April 1, 2016. However, in the case of drugs which are listed in the Pharmacopoeia (hereinafter referred to as ``previ- ous Pharmacopoeia'') [limited to those listed in the Japanese Pharmacopoeia whose standards are changed in accordance with this notification (hereinafter referred to as ``new Pharmacopoeia'')] and have been approved as of April 1, 2016 as prescribed under Paragraph 1, Article 14 of the same law [including drugs the Minister of Health, Labour and Welfare specifies (the Ministry of Health and Welfare Ministerial Notification No. 104, 1994) as of March 31, 2016 as those exempted from marketing approval pursuant to Paragraph 1, Article 14 of the Same Law (hereinafter referred to as ``drugs exempted from approval'')], the Name and Standards established in the previous Pharmacopoeia (limited to part of the Name and Standards for the drugs concerned) may be accepted to conform to the Name and Standards established in the new Pharmacopoeia before and on September 30, 2017. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Antidote List
Antidote List Recommended minimum amounts for hospital pharmacies to stock Call the Maryland Poison Center for expert advice on the treatment of all poisonings and overdoses: 1-800-222-1222 Poisoning Minimum Stocking Antidote Indication Recommendations Oral: 120 grams Acetylcysteine Acetaminophen IV: 96 grams Crotaline snake Antivenin, snake (CroFab®) 12 vials envenomation 1 vial Black widow spider (Note: Merck limits distribution Antivenin, black widow spider envenomation to cases of confirmed bites with symptoms only) Organophosphate & Atropine carbamate insecticides, nerve 165 mg gases Calcium chloride Calcium channel blockers 10 grams 2.25 grams Calcium disodium EDTA Lead (Available direct from ASD Specialty Healthcare) Hydrofluoric acid 1 kg (powder) Calcium gluconate Calcium channel blockers IV: 30 grams 1 kit Cyanide Antidote Kit Cyanide (or Hydroxocobalamin, see below) Cyproheptadine (Periactin®) Serotonin syndrome 80 mg Neuroleptic malignant Dantrolene syndrome, stimulant-induced 720 mg hyperthermia Deferoxamine mesylate Iron 8 grams (Desferal®) Digoxin Immune FAB Digoxin 20 vials (Digibind®, DigiFab®) Dimercaprol (BAL) Heavy metals 1.5 grams 1 | P a g e Maryland Poison Center Antidote List – continued Poisoning Minimum Stocking Antidote Indication Recommendations DMSA (Succimer, Chemet®) Heavy metals 2000 mg Folic acid Methanol IV: 150 mg Flumazenil (Romazicon®) Benzodiazepines 10 mg Fomepizole (Antizol®) Ethylene glycol, methanol 12 grams Beta blockers, Glucagon 50 mg calcium channel blockers Hydroxocobalamin (Cyanokit®) Cyanide -

Protamine Sulfate Enhances Lipid-Mediated Gene Transfer
Gene Therapy (1997) 4, 961–968 1997 Stockton Press All rights reserved 0969-7128/97 $12.00 Protamine sulfate enhances lipid-mediated gene transfer FL Sorgi1,2, S Bhattacharya1 and L Huang1 1The Laboratory of Drug Targeting, Department of Pharmacology, The University of Pittsburgh School of Medicine, Pittsburgh, PA, USA A polycationic peptide, protamine sulfate, USP, has been USP as a condensation agent was found to be superior to shown to be able to condense plasmid DNA efficiently for poly-L-lysine as well as to various other types of protamine. delivery into several different types of cells in vitro by sev- These differences among various salt forms of protamine eral different types of cationic liposomes. The monovalent appear to be attributable to structural differences between cationic liposomal formulations (DC-Chol and lipofectin) the protamines and not due to differences in the net charge exhibited increased transfection activities comparable to of the molecule. The appearance of lysine residues within that seen with the multivalent cationic liposome formu- the protamine molecule correlate with a reduction in bind- lation, lipofectamine. This suggests that lipofectamine’s ing affinity to plasmid DNA, as well as an observed loss in superior in vitro activity arises from its ability to condense transfection-enhancing activity. This finding sheds light on DNA efficiently and that protamine’s primary role is that of the structural requirements of condensation agents for use a condensation agent, although it also possesses several in gene transfer protocols. Furthermore, protamine sulfate, amino acid sequences resembling that of a nuclear localiz- USP is an FDA-approved compound with a documented ation signal.