The Selection of Essential Drugs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Surfen, a Small Molecule Antagonist of Heparan Sulfate
Surfen, a small molecule antagonist of heparan sulfate Manuela Schuksz*†, Mark M. Fuster‡, Jillian R. Brown§, Brett E. Crawford§, David P. Ditto¶, Roger Lawrence*, Charles A. Glass§, Lianchun Wang*, Yitzhak Torʈ, and Jeffrey D. Esko*,** *Department of Cellular and Molecular Medicine, Glycobiology Research and Training Center, †Biomedical Sciences Graduate Program, ‡Department of Medicine, Division of Pulmonary and Critical Care Medicine and Veteran’s Administration San Diego Medical Center, ¶Moores Cancer Center, and ʈDepartment of Chemistry and Biochemistry, University of California at San Diego, La Jolla, CA 92093; and §Zacharon Pharmaceuticals, Inc, 505 Coast Blvd, South, La Jolla, CA 92037 Communicated by Carolyn R. Bertozzi, University of California, Berkeley, CA, June 18, 2008 (received for review May 26, 2007) In a search for small molecule antagonists of heparan sulfate, Surfen (bis-2-methyl-4-amino-quinolyl-6-carbamide) was first we examined the activity of bis-2-methyl-4-amino-quinolyl-6- described in 1938 as an excipient for the production of depot carbamide, also known as surfen. Fluorescence-based titrations insulin (16). Subsequent studies have shown that surfen can indicated that surfen bound to glycosaminoglycans, and the extent block C5a receptor binding (17) and lethal factor (LF) produced of binding increased according to charge density in the order by anthrax (18). It was also reported to have modest heparin- heparin > dermatan sulfate > heparan sulfate > chondroitin neutralizing effects in an oral feeding experiments in rats (19), sulfate. All charged groups in heparin (N-sulfates, O-sulfates, and but to our knowledge, no further studies involving heparin have carboxyl groups) contributed to binding, consistent with the idea been conducted, and its effects on HS are completely unknown. -

Computational Studies of Drug Repurposing Targeting P-Glycoprotein Mediated Multidrug-Resistance Phenotypes in Agents of Neglect
bioRxiv preprint doi: https://doi.org/10.1101/2020.06.12.147926; this version posted June 12, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Computational studies of drug repurposing targeting P- glycoprotein mediated multidrug-resistance phenotypes in agents of neglected tropical diseases Nivedita Jaishankar 1, Sangeetha Muthamilselvan 2, Ashok Palaniappan 1,2* 1 Department of Biotechnology, Sri Venkateswara College of Engineering, Post Bag No. 1, Pennalur, Sriperumbudur Tk 602117. India 2 Department of Bioinformatics, School of Chemical and BioTechnology, SASTRA Deemed University, Thanjavur 613401. India * Corresponding author: [email protected] ABSTRACT Mammalian ABCB1 P-glycoprotein is an ATP- dependent efflux pump with broad substrate specificity associated with cellular drug resistance. Homologous to this role in mammalian biology, the P-glycoprotein of agents of neglected tropical diseases (NTDs) mediates the emergence of multidrug- resistance phenotypes. The clinical and socioeconomic implications of NTDs are exacerbated by the lack of research interest among Big Pharma for treating such conditions. This work aims to characterise P-gp homologues in certain agents of key NTDs, namely (1) Protozoa: Leishmania major, Trypanosoma cruzi; (2) Helminths: Onchocerca volvulus, Schistosoma mansoni. PSI-BLAST searches against the genome of each of these organisms confirmed the presence of P-gp homologues. Each homologue was aligned against five P- gp sequences of known structure, to identify the most suitable template based on sequence homology, phylogenetic nearest neighbor, and query coverage. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

WO 2012/148799 Al 1 November 2012 (01.11.2012) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2012/148799 Al 1 November 2012 (01.11.2012) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 9/107 (2006.01) A61K 9/00 (2006.01) kind of national protection available): AE, AG, AL, AM, A 61 47/10 (2006.0V) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, PCT/US2012/034361 HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, (22) International Filing Date: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 20 April 2012 (20.04.2012) MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (26) Publication Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/480,259 28 April 201 1 (28.04.201 1) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, (71) Applicant (for all designated States except US): BOARD UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, OF REGENTS, THE UNIVERSITY OF TEXAS SYS¬ TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, TEM [US/US]; 201 West 7th St., Austin, TX 78701 (US). -

Revista Brasileira De Anestesiologia
Rev BrasBras Anestesiol. Anestesiol. 2013;63(1):163–164 2013;63(1):163-166 REVISTA BRASILEIRA DE ANESTESIOLOGIA Offi cial Publication of the Brazilian Society of Anesthesiology www.sba.com.br/rba/index.asp LETTER TO THE EDITOR Precipitation in Gallipoli: Sugammadex / Amiodarone & Sugammadex / Dobutamine & Sugammadex / Protamine Dear Editor, furosemide (10 mg.mL-1), gentamicin (40 mg.mL-1), glyceryl Sugammadex is a modifi ed gamma cyclodextrin 1-3. Cyclodextrins trinitrate (5 mg.mL-1), heparin (1,000 IU.mL-1), hydrocor- are water soluble cyclic oligosaccharides with a lipophilic tisone (250 mg.mL-1), crystallized insulin (100 IU.mL-1), core. Sugammadex has quickly found a place in clinical use Calcium (Calcium Gluconate Monohydrate 225 mg.10 mL-1 for selective antagonism of neuromuscular blockade with ro- + Calcium levulinate dihitrate 572 mg.10 mL-1), ketamine curonium 1-3. Sugammadex quickly encapsulates steroidal neu- (50 mg.mL-1), levobupivacaine (7.5 mg.mL-1), magnesium romuscular blockers, increasing the amount of encapsulated sulphate (1.2 mEq.mL-1), metamizol sodium (0.5 g.mL-1), steroidal neuromuscular blockers in plasma and separating the methylergobasin maleate (0.2 mg.mL-1), metoclopramide blockers from the nicotinic acetylcholine receptors 1-3. (5 mg.mL-1), metoprolol (1 mg.mL-1), morphine (0.01 g.mL-1), Apart from its use with steroidal neuromuscular block- midazolam (5 mg.mL-1), n-acetylcysteine (100 mg.mL-1), ers, it is known that sugammadex interacts with over 40 naloxone (0.4 mg.mL-1), neostigmine (0.5 mg.mL-1), nitroprus- lipophilic, steroidal and non-steroidal drugs. -
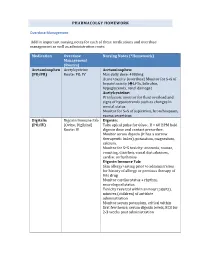
PHARMACOLGY HOMEWORK Overdose Management Add In
PHARMACOLGY HOMEWORK Overdose Management Add in important nursing notes for each of these medications and overdose management as well as administration route. Medication Overdose Nursing Notes (*Homework) Management (Routes) Acetaminophen Acetylcysteine Acetaminophen: (PO/PR) Route: PO, IV Max daily dose: 4000mg Acute toxicity (overdose) Monitor for S+S of hepatotoxicity (éLFTs, bilirubin, hypoglycemia, renal damage) Acetylcysteine: IV infusion: monitor for fluid overload and signs of hyponatremia such as changes in mental status Monitor for S+S of aspiration, bronchospasm, excess secretions Digitalis Digoxin Immune Fab Digoxin: (PO/IV) (Ovine, Digibind) Take apical pulse for 60sec. If < 60 BPM hold Route: IV digoxin dose and contact prescriber. Monitor serum digoxin (it has a narrow therapeutic index), potassium, magnesium, calcium. Monitor for S+S toxicity: anorexia, nausea, vomiting, diarrhea, visual disturbances, cardiac arrhythmias Digoxin Immune Fab: Skin allergy testing prior to administration for history of allergy or previous therapy of this drug Monitor cardiac status + rhythm, neurological status Toxicity reversal within an hour (adults), minutes (children) of antidote administration Monitor serum potassium, critical within first few hours; serum digoxin levels, ECG for 2-3 weeks post administration Heparin Protamine sulfate Heparin: (IV/SC) (IV) Monitor for spontaneous bleeding, thrombocytopenia Monitor aPTT levels Protamine Sulfate: Sudden drop in BP Monitor BP+ P q15-30 min Monitor aPTT Opioid Naloxone (IV-adults, Opioids: -

Comparative Genomics of the Major Parasitic Worms
Comparative genomics of the major parasitic worms International Helminth Genomes Consortium Supplementary Information Introduction ............................................................................................................................... 4 Contributions from Consortium members ..................................................................................... 5 Methods .................................................................................................................................... 6 1 Sample collection and preparation ................................................................................................................. 6 2.1 Data production, Wellcome Trust Sanger Institute (WTSI) ........................................................................ 12 DNA template preparation and sequencing................................................................................................. 12 Genome assembly ........................................................................................................................................ 13 Assembly QC ................................................................................................................................................. 14 Gene prediction ............................................................................................................................................ 15 Contamination screening ............................................................................................................................ -

Protamine Characterization by Top-Down Proteomics: Boosting Proteoform Identification with DBSCAN
proteomes Article Protamine Characterization by Top-Down Proteomics: Boosting Proteoform Identification with DBSCAN Gianluca Arauz-Garofalo 1, Meritxell Jodar 2,3 , Mar Vilanova 1, Alberto de la Iglesia Rodriguez 2, Judit Castillo 2 , Ada Soler-Ventura 2, Rafael Oliva 2,3,* , Marta Vilaseca 1,* and Marina Gay 1,* 1 Institute for Research in Biomedicine (IRB Barcelona), The Barcelona Institute of Science and Technology, Baldiri Reixac, 10, 08028 Barcelona, Spain; [email protected] (G.A.-G.); [email protected] (M.V.) 2 Molecular Biology of Reproduction and Development Research Group, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Fundació Clínic per a la Recerca Biomèdica (FCRB), Faculty of Medicine and Health Sciences, University of Barcelona, 08036 Barcelona, Spain; [email protected] (M.J.); [email protected] (A.d.l.I.R.); [email protected] (J.C.); [email protected] (A.S.-V.) 3 Biochemistry and Molecular Genetics Service, Hospital Clínic de Barcelona, 08036 Barcelona, Spain * Correspondence: [email protected] (R.O.); [email protected] (M.V.); [email protected] (M.G.) Abstract: Protamines replace histones as the main nuclear protein in the sperm cells of many species and play a crucial role in compacting the paternal genome. Human spermatozoa contain protamine 1 (P1) and the family of protamine 2 (P2) proteins. Alterations in protamine PTMs or the P1/P2 ratio Citation: Arauz-Garofalo, G.; may be associated with male infertility. Top-down proteomics enables large-scale analysis of intact Jodar, M.; Vilanova, M.; proteoforms derived from alternative splicing, missense or nonsense genetic variants or PTMs. -

I Comparative Activity of Anthelmintic
Retour au menu Comparative activity of anthelmintic drugs, mebendazole, praziquantel and albendazole against Hymenolepis M. Gala1 ’ diminufa in experimentally infected S. R. Chin ’ Irats GALAL (MT), CHIN (S. R.). Comparaison de l’action de différents CAVIER and ROSSIGNOL (3) studied the taenicidal anthelminttuques (mébendazole, praziquantel et albendazole) contre properties of albendazole, mebendazole, niclosamide Hymenoleph diminuta chez des rats expérimentalement Infectés. Rev. Elev. Méd. vét. Pays trop., 1987, 40 (2) : 147-150. and praziquantel in experimentally infected mice and found that praziquantel and niclosamide have similar Des rats expérimentalement infectés avec If. diminufa ont été traités par voie orale avec trois anthelminthiques, administrés soit en dose taenicidal propet-ties. unique, soit en tiers de dose 3 jours consécutifs. Le praziquantel (250 mgkg ou 83 mg/k&j 3 fois) et I’alhendazole (800 mgkg ou Now are reported the comparative efficacy of meben- 167 mgkg/j 3 fois) ont totalement éliminé les vers, alors que le dazole, praziquantel and albendazole against experi- mébendazole (500 rng/kg- - ou 167 mg/kg/i- -. 3 fois) ne les a aue oartielle- mental infection of rats with H. diminuta, using single ment éliminés, avec une efficacité de 76 p. 160. Parmi ces j anthel- and divided oral doses for 3 consecutive days, 30 days mlnthlques, il n’a pas été relevé de différence signiiïcative d’efkacité entre les différents dosages pour chaque traitement. Aucune toxicité after infection. n’est apparue chez les rats traités. Mots CES : Rat - Helminthosc - Hymenolepis diminuta - Anthelminthique - Mébendazole - Prazi- quantel - Albendazole - Infection expérimentale. MATERIALS AND METHODS INTRODUCTION Definitive and intermediate hosts Albino rats (Rattus norvegicus) of both sexes and H. -

Jp Xvii the Japanese Pharmacopoeia
JP XVII THE JAPANESE PHARMACOPOEIA SEVENTEENTH EDITION Official from April 1, 2016 English Version THE MINISTRY OF HEALTH, LABOUR AND WELFARE Notice: This English Version of the Japanese Pharmacopoeia is published for the convenience of users unfamiliar with the Japanese language. When and if any discrepancy arises between the Japanese original and its English translation, the former is authentic. The Ministry of Health, Labour and Welfare Ministerial Notification No. 64 Pursuant to Paragraph 1, Article 41 of the Law on Securing Quality, Efficacy and Safety of Products including Pharmaceuticals and Medical Devices (Law No. 145, 1960), the Japanese Pharmacopoeia (Ministerial Notification No. 65, 2011), which has been established as follows*, shall be applied on April 1, 2016. However, in the case of drugs which are listed in the Pharmacopoeia (hereinafter referred to as ``previ- ous Pharmacopoeia'') [limited to those listed in the Japanese Pharmacopoeia whose standards are changed in accordance with this notification (hereinafter referred to as ``new Pharmacopoeia'')] and have been approved as of April 1, 2016 as prescribed under Paragraph 1, Article 14 of the same law [including drugs the Minister of Health, Labour and Welfare specifies (the Ministry of Health and Welfare Ministerial Notification No. 104, 1994) as of March 31, 2016 as those exempted from marketing approval pursuant to Paragraph 1, Article 14 of the Same Law (hereinafter referred to as ``drugs exempted from approval'')], the Name and Standards established in the previous Pharmacopoeia (limited to part of the Name and Standards for the drugs concerned) may be accepted to conform to the Name and Standards established in the new Pharmacopoeia before and on September 30, 2017. -

Redalyc.Determination of Niclosamide and Its Metabolites in Liver And
Acta Scientiae Veterinariae ISSN: 1678-0345 [email protected] Universidade Federal do Rio Grande do Sul Brasil Kartalovic, Brankica; Pucarevic, Mira; Markovic, Zoran; Stankovic, Marko; Novakov, Nikolina; Pelic, Milos; Cirkovic, Miroslav Determination of Niclosamide and its Metabolites in Liver and Muscles of Common Carp (Cyprinus carpio) Fingerlings Acta Scientiae Veterinariae, vol. 45, 2017, pp. 1-6 Universidade Federal do Rio Grande do Sul Porto Alegre, Brasil Available in: http://www.redalyc.org/articulo.oa?id=289053641022 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Acta Scientiae Veterinariae, 2017. 45: 1490. RESEARCH ARTICLE ISSN 1679-9216 Pub. 1490 Determination of Niclosamide and its Metabolites in Liver and Muscles of Common Carp (Cyprinus carpio) Fingerlings Brankica Kartalović1 , Mira Pucarević2, Zoran Marković3, Marko Stanković3, Nikolina Novakov4, Milos Pelić1 & Miroslav Ćirković1 ABSTRACT Background: Niclosamide is a medication used to treat tapeworm infestation in animals and humans. It is also lampricide and molluscicide, and can be used in in agriculture as a pesticide. In the treatment of parasitic diseases in fish, niclosamide can be used as bath or mixed with the feed. Its most important use in common carp (Cyprinus carpio) is for the treatment of Bothriocephalus acheilognathi, which is a very common parasite in this fish species. The aim of this study was to de- termine the concentrations of niclosamide (NIC) and its metabolite 2-chloro 4-nitro aniline (CNA) and 5-chloro salycilic acid (CSA) in the liver and muscles of common carp fingerlings. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole