Protamine Sulfate Enhances Lipid-Mediated Gene Transfer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Surfen, a Small Molecule Antagonist of Heparan Sulfate
Surfen, a small molecule antagonist of heparan sulfate Manuela Schuksz*†, Mark M. Fuster‡, Jillian R. Brown§, Brett E. Crawford§, David P. Ditto¶, Roger Lawrence*, Charles A. Glass§, Lianchun Wang*, Yitzhak Torʈ, and Jeffrey D. Esko*,** *Department of Cellular and Molecular Medicine, Glycobiology Research and Training Center, †Biomedical Sciences Graduate Program, ‡Department of Medicine, Division of Pulmonary and Critical Care Medicine and Veteran’s Administration San Diego Medical Center, ¶Moores Cancer Center, and ʈDepartment of Chemistry and Biochemistry, University of California at San Diego, La Jolla, CA 92093; and §Zacharon Pharmaceuticals, Inc, 505 Coast Blvd, South, La Jolla, CA 92037 Communicated by Carolyn R. Bertozzi, University of California, Berkeley, CA, June 18, 2008 (received for review May 26, 2007) In a search for small molecule antagonists of heparan sulfate, Surfen (bis-2-methyl-4-amino-quinolyl-6-carbamide) was first we examined the activity of bis-2-methyl-4-amino-quinolyl-6- described in 1938 as an excipient for the production of depot carbamide, also known as surfen. Fluorescence-based titrations insulin (16). Subsequent studies have shown that surfen can indicated that surfen bound to glycosaminoglycans, and the extent block C5a receptor binding (17) and lethal factor (LF) produced of binding increased according to charge density in the order by anthrax (18). It was also reported to have modest heparin- heparin > dermatan sulfate > heparan sulfate > chondroitin neutralizing effects in an oral feeding experiments in rats (19), sulfate. All charged groups in heparin (N-sulfates, O-sulfates, and but to our knowledge, no further studies involving heparin have carboxyl groups) contributed to binding, consistent with the idea been conducted, and its effects on HS are completely unknown. -

Revista Brasileira De Anestesiologia
Rev BrasBras Anestesiol. Anestesiol. 2013;63(1):163–164 2013;63(1):163-166 REVISTA BRASILEIRA DE ANESTESIOLOGIA Offi cial Publication of the Brazilian Society of Anesthesiology www.sba.com.br/rba/index.asp LETTER TO THE EDITOR Precipitation in Gallipoli: Sugammadex / Amiodarone & Sugammadex / Dobutamine & Sugammadex / Protamine Dear Editor, furosemide (10 mg.mL-1), gentamicin (40 mg.mL-1), glyceryl Sugammadex is a modifi ed gamma cyclodextrin 1-3. Cyclodextrins trinitrate (5 mg.mL-1), heparin (1,000 IU.mL-1), hydrocor- are water soluble cyclic oligosaccharides with a lipophilic tisone (250 mg.mL-1), crystallized insulin (100 IU.mL-1), core. Sugammadex has quickly found a place in clinical use Calcium (Calcium Gluconate Monohydrate 225 mg.10 mL-1 for selective antagonism of neuromuscular blockade with ro- + Calcium levulinate dihitrate 572 mg.10 mL-1), ketamine curonium 1-3. Sugammadex quickly encapsulates steroidal neu- (50 mg.mL-1), levobupivacaine (7.5 mg.mL-1), magnesium romuscular blockers, increasing the amount of encapsulated sulphate (1.2 mEq.mL-1), metamizol sodium (0.5 g.mL-1), steroidal neuromuscular blockers in plasma and separating the methylergobasin maleate (0.2 mg.mL-1), metoclopramide blockers from the nicotinic acetylcholine receptors 1-3. (5 mg.mL-1), metoprolol (1 mg.mL-1), morphine (0.01 g.mL-1), Apart from its use with steroidal neuromuscular block- midazolam (5 mg.mL-1), n-acetylcysteine (100 mg.mL-1), ers, it is known that sugammadex interacts with over 40 naloxone (0.4 mg.mL-1), neostigmine (0.5 mg.mL-1), nitroprus- lipophilic, steroidal and non-steroidal drugs. -
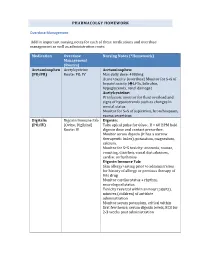
PHARMACOLGY HOMEWORK Overdose Management Add In
PHARMACOLGY HOMEWORK Overdose Management Add in important nursing notes for each of these medications and overdose management as well as administration route. Medication Overdose Nursing Notes (*Homework) Management (Routes) Acetaminophen Acetylcysteine Acetaminophen: (PO/PR) Route: PO, IV Max daily dose: 4000mg Acute toxicity (overdose) Monitor for S+S of hepatotoxicity (éLFTs, bilirubin, hypoglycemia, renal damage) Acetylcysteine: IV infusion: monitor for fluid overload and signs of hyponatremia such as changes in mental status Monitor for S+S of aspiration, bronchospasm, excess secretions Digitalis Digoxin Immune Fab Digoxin: (PO/IV) (Ovine, Digibind) Take apical pulse for 60sec. If < 60 BPM hold Route: IV digoxin dose and contact prescriber. Monitor serum digoxin (it has a narrow therapeutic index), potassium, magnesium, calcium. Monitor for S+S toxicity: anorexia, nausea, vomiting, diarrhea, visual disturbances, cardiac arrhythmias Digoxin Immune Fab: Skin allergy testing prior to administration for history of allergy or previous therapy of this drug Monitor cardiac status + rhythm, neurological status Toxicity reversal within an hour (adults), minutes (children) of antidote administration Monitor serum potassium, critical within first few hours; serum digoxin levels, ECG for 2-3 weeks post administration Heparin Protamine sulfate Heparin: (IV/SC) (IV) Monitor for spontaneous bleeding, thrombocytopenia Monitor aPTT levels Protamine Sulfate: Sudden drop in BP Monitor BP+ P q15-30 min Monitor aPTT Opioid Naloxone (IV-adults, Opioids: -

Protamine Characterization by Top-Down Proteomics: Boosting Proteoform Identification with DBSCAN
proteomes Article Protamine Characterization by Top-Down Proteomics: Boosting Proteoform Identification with DBSCAN Gianluca Arauz-Garofalo 1, Meritxell Jodar 2,3 , Mar Vilanova 1, Alberto de la Iglesia Rodriguez 2, Judit Castillo 2 , Ada Soler-Ventura 2, Rafael Oliva 2,3,* , Marta Vilaseca 1,* and Marina Gay 1,* 1 Institute for Research in Biomedicine (IRB Barcelona), The Barcelona Institute of Science and Technology, Baldiri Reixac, 10, 08028 Barcelona, Spain; [email protected] (G.A.-G.); [email protected] (M.V.) 2 Molecular Biology of Reproduction and Development Research Group, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Fundació Clínic per a la Recerca Biomèdica (FCRB), Faculty of Medicine and Health Sciences, University of Barcelona, 08036 Barcelona, Spain; [email protected] (M.J.); [email protected] (A.d.l.I.R.); [email protected] (J.C.); [email protected] (A.S.-V.) 3 Biochemistry and Molecular Genetics Service, Hospital Clínic de Barcelona, 08036 Barcelona, Spain * Correspondence: [email protected] (R.O.); [email protected] (M.V.); [email protected] (M.G.) Abstract: Protamines replace histones as the main nuclear protein in the sperm cells of many species and play a crucial role in compacting the paternal genome. Human spermatozoa contain protamine 1 (P1) and the family of protamine 2 (P2) proteins. Alterations in protamine PTMs or the P1/P2 ratio Citation: Arauz-Garofalo, G.; may be associated with male infertility. Top-down proteomics enables large-scale analysis of intact Jodar, M.; Vilanova, M.; proteoforms derived from alternative splicing, missense or nonsense genetic variants or PTMs. -

Jp Xvii the Japanese Pharmacopoeia
JP XVII THE JAPANESE PHARMACOPOEIA SEVENTEENTH EDITION Official from April 1, 2016 English Version THE MINISTRY OF HEALTH, LABOUR AND WELFARE Notice: This English Version of the Japanese Pharmacopoeia is published for the convenience of users unfamiliar with the Japanese language. When and if any discrepancy arises between the Japanese original and its English translation, the former is authentic. The Ministry of Health, Labour and Welfare Ministerial Notification No. 64 Pursuant to Paragraph 1, Article 41 of the Law on Securing Quality, Efficacy and Safety of Products including Pharmaceuticals and Medical Devices (Law No. 145, 1960), the Japanese Pharmacopoeia (Ministerial Notification No. 65, 2011), which has been established as follows*, shall be applied on April 1, 2016. However, in the case of drugs which are listed in the Pharmacopoeia (hereinafter referred to as ``previ- ous Pharmacopoeia'') [limited to those listed in the Japanese Pharmacopoeia whose standards are changed in accordance with this notification (hereinafter referred to as ``new Pharmacopoeia'')] and have been approved as of April 1, 2016 as prescribed under Paragraph 1, Article 14 of the same law [including drugs the Minister of Health, Labour and Welfare specifies (the Ministry of Health and Welfare Ministerial Notification No. 104, 1994) as of March 31, 2016 as those exempted from marketing approval pursuant to Paragraph 1, Article 14 of the Same Law (hereinafter referred to as ``drugs exempted from approval'')], the Name and Standards established in the previous Pharmacopoeia (limited to part of the Name and Standards for the drugs concerned) may be accepted to conform to the Name and Standards established in the new Pharmacopoeia before and on September 30, 2017. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Antidote List
Antidote List Recommended minimum amounts for hospital pharmacies to stock Call the Maryland Poison Center for expert advice on the treatment of all poisonings and overdoses: 1-800-222-1222 Poisoning Minimum Stocking Antidote Indication Recommendations Oral: 120 grams Acetylcysteine Acetaminophen IV: 96 grams Crotaline snake Antivenin, snake (CroFab®) 12 vials envenomation 1 vial Black widow spider (Note: Merck limits distribution Antivenin, black widow spider envenomation to cases of confirmed bites with symptoms only) Organophosphate & Atropine carbamate insecticides, nerve 165 mg gases Calcium chloride Calcium channel blockers 10 grams 2.25 grams Calcium disodium EDTA Lead (Available direct from ASD Specialty Healthcare) Hydrofluoric acid 1 kg (powder) Calcium gluconate Calcium channel blockers IV: 30 grams 1 kit Cyanide Antidote Kit Cyanide (or Hydroxocobalamin, see below) Cyproheptadine (Periactin®) Serotonin syndrome 80 mg Neuroleptic malignant Dantrolene syndrome, stimulant-induced 720 mg hyperthermia Deferoxamine mesylate Iron 8 grams (Desferal®) Digoxin Immune FAB Digoxin 20 vials (Digibind®, DigiFab®) Dimercaprol (BAL) Heavy metals 1.5 grams 1 | P a g e Maryland Poison Center Antidote List – continued Poisoning Minimum Stocking Antidote Indication Recommendations DMSA (Succimer, Chemet®) Heavy metals 2000 mg Folic acid Methanol IV: 150 mg Flumazenil (Romazicon®) Benzodiazepines 10 mg Fomepizole (Antizol®) Ethylene glycol, methanol 12 grams Beta blockers, Glucagon 50 mg calcium channel blockers Hydroxocobalamin (Cyanokit®) Cyanide -

Reducing the Use of Reversal Agents in a Community Hospital
4/21/2016 Objectives • Review the appropriate use of reversal agents Reducing the Use of Reversal Agents in a Community Hospital • Identify opportunities for reduction strategies of reversal agents Maria Paulina Duarte, PharmD PGY-1 Pharmacy Resident • Understand pharmacist’s role in impacting the Mercy Hospital, A Campus of use of reversal agents Plantation General Hospital Background Reversal Agents • Drug overdoses, whether accidental or intentional, • Naloxone constitute a significant source of increasing ▫ Reversal of opioids morbidity, mortality, and healthcare expenditure Examples: Morphine, hydromorphone, and fentanyl worldwide1,2 ▫ Side effects: Cardiac complications • Flumazenil • Hospitalized patients are susceptible to accidental ▫ Reversal of benzodiazepines drug overdoses, and immediate reversal may be 3 Examples: Lorazepam, clonazepam, and diazepam warranted to decrease harm ▫ Side effects: May result in seizures • Vitamin K • However, in some clinical settings, it has become ▫ Reversal of vitamin K antagonist common practice to use reversal agents and in some Example: Warfarin occasions the use of reversal agents might not be of ▫ Side effects: May cause warfarin resistance value for a drug overdose3,4 Reversal Agents Study’s Objective • Protamine ▫ Reversal of heparin Example: Unfractionated heparin and low molecular To assess the utilization of reversal agents weight heparin ▫ Side effects: cardiac and pulmonary complications and identify areas to potentially streamline their use • Prothrombin Complex Concentrate -

World Health Organization Model List of Essential Medicines, 21St List, 2019
World Health Organizatio n Model List of Essential Medicines 21st List 2019 World Health Organizatio n Model List of Essential Medicines 21st List 2019 WHO/MVP/EMP/IAU/2019.06 © World Health Organization 2019 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. World Health Organization Model List of Essential Medicines, 21st List, 2019. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. CIP data are available at http://apps.who.int/iris. -

Use of Protamine in Nanopharmaceuticals—A Review
nanomaterials Review Use of Protamine in Nanopharmaceuticals—A Review Ivana Ruseska †, Katja Fresacher †, Christina Petschacher and Andreas Zimmer * Department of Pharmaceutical Technology and Biopharmacy, Institute of Pharmaceutical Sciences, Karl-Franzens-University Graz, Universitätsplatz 1, 8010 Graz, Austria; [email protected] (I.R.); [email protected] (K.F.); [email protected] (C.P.) * Correspondence: [email protected] † Both authors are contributed equally. Abstract: Macromolecular biomolecules are currently dethroning classical small molecule therapeu- tics because of their improved targeting and delivery properties. Protamine-a small polycationic peptide-represents a promising candidate. In nature, it binds and protects DNA against degradation during spermatogenesis due to electrostatic interactions between the negatively charged DNA- phosphate backbone and the positively charged protamine. Researchers are mimicking this technique to develop innovative nanopharmaceutical drug delivery systems, incorporating protamine as a carrier for biologically active components such as DNA or RNA. The first part of this review high- lights ongoing investigations in the field of protamine-associated nanotechnology, discussing the self-assembling manufacturing process and nanoparticle engineering. Immune-modulating prop- erties of protamine are those that lead to the second key part, which is protamine in novel vaccine technologies. Protamine-based RNA delivery systems in vaccines (some belong to the new class of mRNA-vaccines) against infectious disease and their use in cancer treatment are reviewed, and we provide an update on the current state of latest developments with protamine as pharmaceutical Citation: Ruseska, I.; Fresacher, K.; excipient for vaccines. Petschacher, C.; Zimmer, A. Use of Protamine in Nanopharmaceuticals— Keywords: protamine; proticles; nanoparticles; novel vaccine technologies A Review. -

Essential Medications Review
Essential medications for high-quality patient care Quarter 3 2021 update Essential medications – September 2021 As part of the mission to end drug shortages, the Vizient team of pharmacy experts continues to identify essential medications where, if not available, would prove the greatest threat to hospitals’ ability to provide immediate and high-quality patient care. Medications identified as of greatest importance were selected by the Vizient pharmacy team from a comprehensive clinical review of products contained within the World Health Organization’s (WHO) Essential Medicines list, the Advanced Cardiac Life Support (ACLS) and Pediatric Advanced Life Support (PALS) algorithms, and medications included in Vizient member health systems’ critical drug lists. As of this edition, 14 drugs were added to the list creating a total of 251 line items, representing 237 unique drugs and three categories. This includes: • Acute treatment drugs with no alternatives (77 drugs) – Medicines used in acute and critical circumstances to sustain life and for which there are no current alternatives • Chronic treatment drugs with no alternatives (37 drugs) – Products used in chronic disease states or conditions where no alternatives are available (e.g., chemotherapy medications) • High impact drugs (137 drugs) – Medicines for which alternatives are available but may be less clinically desirable and/or are more operationally difficult to use; also reflects drugs where the absence of one medication can affect therapeutically related drugs. Updated quarterly, -

Poison Control Antidote Poster
Antidote Chart (The suggested minimum stocking level is a combination of factors; anticipation of the highest total dose of a drug generally given during a 24 hour period to a 70 kg adult.) General Decontamination Uses Dose Comments Activated Charcoal (without sorbitol) Most ingestions occurring within one hour Adults: 25–100 grams, Children: 1 g/kg May be given in multiple doses depending on ingestant to enhance elimination Activated Charcoal (with sorbitol) May be used as first AC given to patient if presents within Adults: 25–50 grams Should not be used for multiple dose activated charcoal regimens one hour of ingestion Children: Not generally recommended Whole Bowel Irrigation Drugs not bound by charcoal, sustained Adults: 500–2000 ml/hour Nasogastric tube should be used to maintain amount given (Polyethylene Glycol) release formulations, and body stuffers Children: 25 ml/kg/hour Syrup of Ipecac No longer recommended No longer recommended No longer recommended Poisoning Antidote Quantity to Stock* Comments Acetaminophen N-ACETYLYCYSTEINE 10–20% 600 ml (20% Mucomyst®) Because vomiting of the oral NAC is common, the facility should maintain a repeat dose (Mucomyst®) or 1200 ml (10% Mucomyst®) for each patient for initial dosing if continuation of treatment at facility is not anticipated. ACETADOTE® The IV Acetadote® was just approved in February 2004. Call the MAPCC for dosing, ACETYLCYSTEINE Injection for IV use 4 (30 ml) vials of 20% solution precautions and contraindications. Both the oral and IV forms of acetylcysteine should be administered within 8 hours for maximal protection against hepatic injury. Anticholinergic Poisoning PHYSOSTIGMINE (Antilirium®) 2–4 mg* Not generally recommended for children.