Volume 1, Chapter 3-1: Sexuality: Sexual Strategies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Contributions to the Moss Flora of the Caucasian Part (Artvin Province) of Turkey
Turkish Journal of Botany Turk J Bot (2013) 37: 375-388 http://journals.tubitak.gov.tr/botany/ © TÜBİTAK Research Article doi:10.3906/bot-1201-49 Contributions to the moss flora of the Caucasian part (Artvin Province) of Turkey 1 2, Nevzat BATAN , Turan ÖZDEMİR * 1 Maçka Vocational School, Karadeniz Technical University, 61750, Trabzon, Turkey 2 Department of Biology, Faculty of Science, Karadeniz Technical University, 61080, Trabzon, Turkey Received: 27.01.2012 Accepted: 02.10.2012 Published Online: 15.03.2013 Printed: 15.04.2013 Abstract: The moss flora of Artvin Province (Ardanuç, Şavşat, Borçka, Murgul, and Arhavi districts) in Turkey was studied between 2009 and 2011. A total of 167 moss taxa (belonging to 80 genera and 33 families) were recorded within the study area. Among these, 3 species [Dicranella schreberiana (Hedw.) Dixon, Dicranodontium asperulum (Mitt.) Broth., and Campylopus pyriformis (Schultz) Brid.] are new records from the investigated area for the moss flora of Turkey. The research area is located in the A4 and A5 squares in the grid system adopted by Henderson in 1961. In the A5 grid-square 127 taxa were recorded as new records, and 1 taxon [Anomodon longifolius (Schleich. ex Brid.) Hartm.] was recorded for the second time in Turkey. Key words: Moss, flora, Artvin Province, A4 and A5 squares, Turkey 1. Introduction 2008), Campylopus flexuosus (Hedw.) Brid. (Özdemir & The total Turkish bryoflora comprises 773 taxa (species, Uyar, 2008), Scapania paludosa (Müll. Frib.) Müll. Frib. subspecies, and varieties), including 187 genera of (Keçeli et al., 2008), Dicranum flexicaule Brid. (Uyar et Bryophyta and 175 taxa (species, subspecies, and varieties) al., 2008), Sphagnum centrale C.E.O.Jensen (Abay et al., of Marchantiophyta and Anthocerotophyta (Uyar & Çetin, 2009), Orthotrichum callistomum Fisch. -
Species Summary Table
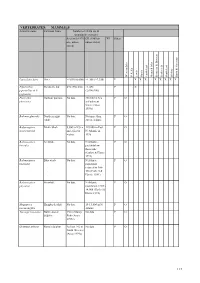
VERTEBRATES: MAMMALS Scientific name Common Name Number of 10 km sqs & (population estimate) Scotland (1970 GB (1960 on WI Status on - unless unless stated) stated) Western Isles St Kilda Lewis Harris North Uist Monach Isles Berneray & Boreray Benbecula South Uist Eriskay Barra & Vatersay Lutra lutra lutra Otter >1,050 (6,600) >1,308 (>7,350) P X X X X X X X X Pipistrellus Pipistrelle bat 492 (550,000) >1,438 P X pipisterllus & P. (2,000,000) pygmaeus Phocoena Harbour porpoise No data 350,000 in Sea P O phocoena and adjacent waters (Anon 1999a) Balaena glacialis Northern right No data Not more than P O whale 300 in Atlantic Balaenoptera Minke whale 8,500 in N Sea 110,000 in East P O acutorostrata and adjacent N. Atlantic in waters 1995 Balaenoptera Sei whale No data N Atlantic - P O borealis probably low thousands (Corbet & Harris 1991) Balaenoptera Blue whale No data N Atlantic P O musculus population reduced to 300- 500 (Corbett & Harris 1991) Balaenoptera Fin whale No data N Atlantic P O physalus population 9,000 - 14,000 (Corbet & Harris 1991) Megaptera Humpback whale No data 10-15,000 in N P O novaeangilea Atlantic Tursiops truncatus Bottle-nosed 130 in Moray No data P O dolphin Firth (Anon 1999a) Grampus griseus Risso's dolphin At least 142 in No data P O North Minches (Anon 1999a) 113 VERTEBRATES: MAMMALS Scientific name Common Name Number of 10 km sqs & (population estimate) Scotland (1970 GB (1960 on WI Status on - unless unless stated) stated) Western Isles St Kilda Lewis Harris North Uist Monach Isles Berneray & Boreray Benbecula -

A Revised Red List of Bryophytes in Britain
ConservationNews Revised Red List distinguished from Extinct. This Red List uses Extinct in the Wild (EW) – a taxon is Extinct version 3.1 of the categories and criteria (IUCN, in the Wild when it is known to survive only in A revised Red List of 2001), along with guidelines produced to assist cultivation or as a naturalized population well with their interpretation and use (IUCN, 2006, outside the past range. There are no taxa in this 2008), further guidelines for using the system category in the British bryophyte flora. bryophytes in Britain at a regional level (IUCN, 2003), and specific Regionally Extinct (RE) – a taxon is regarded guidelines for applying the system to bryophytes as Regionally Extinct in Britain if there are no (Hallingbäck et al., 1995). post-1979 records and all known localities have Conservation OfficerNick Hodgetts presents the latest revised Red List for How these categories and criteria have been been visited and surveyed without success, or interpreted and applied to the British bryophyte if colonies recorded post-1979 are known to bryophytes in Britain. Dumortiera hirsuta in north Cornwall. Ian Atherton flora is summarized below, but anyone interested have disappeared. It should be appreciated that in looking into them in more depth should regional ‘extinction’ for bryophytes is sometimes he first published Red List of et al. (2001) and Preston (2010), varieties and consult the original IUCN documents, which less final than for other, more conspicuous bryophytes in Britain was produced subspecies have been disregarded. are available on the IUCN website (www. organisms. This may be because bryophytes are in 2001 as part of a Red Data Book 1980 has been chosen as the cut-off year to iucnredlist.org/technical-documents/categories- easily overlooked, or because their very efficient for bryophytes (Church et al., 2001). -

Biodiverse Master
Montane, Heath and Bog Habitats MONTANE, HEATH AND BOG HABITATS CONTENTS Montane, heath and bog introduction . 66 Opportunities for action in the Cairngorms . 66 The main montane, heath and bog biodiversity issues . 68 Main threats to UK montane, heath and bog Priority species in the Cairngorms . 72 UK Priority species and Locally important species accounts . 73 Cairngorms montane, heath and bog habitat accounts: • Montane . 84 • Upland heath . 87 • Blanket bog . 97 • Raised bog . 99 ‘Key’ Cairngorms montane, heath and bog species . 100 65 The Cairngorms Local Biodiversity Action Plan MONTANE, HEATH AND BOG INTRODUCTION Around one third of the Cairngorms Partnership area is over 600-650m above sea level (above the natural woodland line, although this is variable from place to place.). This comprises the largest and highest area of montane habitat in Britain, much of which is in a relatively pristine condition. It contains the main summits and plateaux with their associated corries, rocky cliffs, crags, boulder fields, scree slopes and the higher parts of some glens and passes. The vegeta- tion is influenced by factors such as exposure, snow cover and soil type. The main zone is considered to be one of the most spectacular mountain areas in Britain and is recognised nationally and internationally for the quality of its geology, geomorphology and topographic features, and associated soils and biodiversity. c14.5% of the Cairngorms Partnership area (75,000ha) is land above 600m asl. Upland heathland is the most extensive habitat type in the Cairngorms Partnership area, covering c41% of the area, frequently in mosaics with blanket bog. -

Fossil Mosses: What Do They Tell Us About Moss Evolution?
Bry. Div. Evo. 043 (1): 072–097 ISSN 2381-9677 (print edition) DIVERSITY & https://www.mapress.com/j/bde BRYOPHYTEEVOLUTION Copyright © 2021 Magnolia Press Article ISSN 2381-9685 (online edition) https://doi.org/10.11646/bde.43.1.7 Fossil mosses: What do they tell us about moss evolution? MicHAEL S. IGNATOV1,2 & ELENA V. MASLOVA3 1 Tsitsin Main Botanical Garden of the Russian Academy of Sciences, Moscow, Russia 2 Faculty of Biology, Lomonosov Moscow State University, Moscow, Russia 3 Belgorod State University, Pobedy Square, 85, Belgorod, 308015 Russia �[email protected], https://orcid.org/0000-0003-1520-042X * author for correspondence: �[email protected], https://orcid.org/0000-0001-6096-6315 Abstract The moss fossil records from the Paleozoic age to the Eocene epoch are reviewed and their putative relationships to extant moss groups discussed. The incomplete preservation and lack of key characters that could define the position of an ancient moss in modern classification remain the problem. Carboniferous records are still impossible to refer to any of the modern moss taxa. Numerous Permian protosphagnalean mosses possess traits that are absent in any extant group and they are therefore treated here as an extinct lineage, whose descendants, if any remain, cannot be recognized among contemporary taxa. Non-protosphagnalean Permian mosses were also fairly diverse, representing morphotypes comparable with Dicranidae and acrocarpous Bryidae, although unequivocal representatives of these subclasses are known only since Cretaceous and Jurassic. Even though Sphagnales is one of two oldest lineages separated from the main trunk of moss phylogenetic tree, it appears in fossil state regularly only since Late Cretaceous, ca. -

Population Ecology of Eriophorum Latifolium, a Clonal Species in Rich Fen Vegetation
Anders Lyngstad Population Ecology of Eriophorum latifolium, a Clonal Species in Rich Fen Vegetation Thesis for the degree of Philosophiae Doctor Trondheim, October 2010 Norwegian University of Science and Technology Faculty of Natural Sciences and Technology Department of Biology NTNU Norwegian University of Science and Technology Thesis for the degree of Philosophiae Doctor Faculty of Natural Sciences and Technology Department of Biology © Anders Lyngstad ISBN 978-82-471-2332-4 (printed ver.) ISBN 978-82-471-2333-1 (electronic ver.) ISSN 1503-8181 Doctoral theses at NTNU, 2010:179 Printed by NTNU-trykk Synopsis PREFACE It was the spring of 2005, and it was the right time to move onwards. A position as a research fellow working with long-term time series at the Museum of Natural History and Archaeology at the Norwegian University of Science and Technology (NTNU) was announced, and a PhD-project was developed with the studies of former haymaking lands at Sølendet and Tågdalen nature reserves as a starting point. Work began in earnest in January 2006, and continued at an ever increasing pace until July 2010, when all the parts of the thesis were finally completed and assembled. The study was financed by NTNU, and was carried out at the Museum of Natural History and Archaeology and the Institute of Biology, both NTNU. I am deeply grateful to my main supervisor Professor Asbjørn Moen at the Museum of Natural History and Archaeology, and my co-supervisor Associate Professor Bård Pedersen at the Institute of Biology. This project rests on the long-term studies of rich hayfens that were initiated by Asbjørn 40 years ago, and that are still ongoing, much due to his continuous effort. -

Anomalies in Male Receptacles of Plagiochasma Appendiculatum Lehm
American International Journal of Available online at http://www.iasir.net Research in Formal, Applied & Natural Sciences ISSN (Print): 2328-3777, ISSN (Online): 2328-3785, ISSN (CD-ROM): 2328-3793 AIJRFANS is a refereed, indexed, peer-reviewed, multidisciplinary and open access journal published by International Association of Scientific Innovation and Research (IASIR), USA (An Association Unifying the Sciences, Engineering, and Applied Research) Anomalies in male receptacles of Plagiochasma appendiculatum Lehm. & Lindenb Pallvi Sharma and Anima Langer Department of Botany, University of Jammu, Jammu, Jammu and Kashmir-180006, INDIA Abstract: Plagiochasma appendiculatum Lehm. & Lindenb. belongs to the family Aytoniaceae which includes five genera (Plagiochasma, Reboulia, Asterella, Mannia and Cryptomitrium). Most of these genera show abnormalities in the development of reproductive structures. In the present paper, anomalies in the morphology and anatomy of male receptacles are reported in P.appendiculatum. Keywords: Plagiochasma appendiculatum; Aytoniaceae; Anomalies; Male receptacles I. Introduction Plagiochasma appendiculatum is a thalloid hepatic which is dorso-ventrally flattened. The male receptacles are normally horse-shoe shaped, sessile and present on the dorsal surface of the thallus, while the female ones are stalked, variously lobed (usually 3-5 lobed) and present on the main thallus too. Abnormality in their behaviour has been observed by bryologists like Kashyap and Bapna [1,4]. A second forking in the two lobes of androecium of P.appendiculatum is one of the important discovery (Kashyap, 1919). Abnormal female receptacles in the same genus were studied by Bapna and Bhagat [1,2] . Anomalies in the reproductive structures have also been studied in other members of family Aytoniaceae like unusual female receptacles in Asterella blumeana nees and Asterella khasiana [5] and abnormal male receptacles in Reboulia hemispherica [6]. -

Molecular Phylogeny of Chinese Thuidiaceae with Emphasis on Thuidium and Pelekium
Molecular Phylogeny of Chinese Thuidiaceae with emphasis on Thuidium and Pelekium QI-YING, CAI1, 2, BI-CAI, GUAN2, GANG, GE2, YAN-MING, FANG 1 1 College of Biology and the Environment, Nanjing Forestry University, Nanjing 210037, China. 2 College of Life Science, Nanchang University, 330031 Nanchang, China. E-mail: [email protected] Abstract We present molecular phylogenetic investigation of Thuidiaceae, especially on Thudium and Pelekium. Three chloroplast sequences (trnL-F, rps4, and atpB-rbcL) and one nuclear sequence (ITS) were analyzed. Data partitions were analyzed separately and in combination by employing MP (maximum parsimony) and Bayesian methods. The influence of data conflict in combined analyses was further explored by two methods: the incongruence length difference (ILD) test and the partition addition bootstrap alteration approach (PABA). Based on the results, ITS 1& 2 had crucial effect in phylogenetic reconstruction in this study, and more chloroplast sequences should be combinated into the analyses since their stability for reconstructing within genus of pleurocarpous mosses. We supported that Helodiaceae including Actinothuidium, Bryochenea, and Helodium still attributed to Thuidiaceae, and the monophyletic Thuidiaceae s. lat. should also include several genera (or species) from Leskeaceae such as Haplocladium and Leskea. In the Thuidiaceae, Thuidium and Pelekium were resolved as two monophyletic groups separately. The results from molecular phylogeny were supported by the crucial morphological characters in Thuidiaceae s. lat., Thuidium and Pelekium. Key words: Thuidiaceae, Thuidium, Pelekium, molecular phylogeny, cpDNA, ITS, PABA approach Introduction Pleurocarpous mosses consist of around 5000 species that are defined by the presence of lateral perichaetia along the gametophyte stems. Monophyletic pleurocarpous mosses were resolved as three orders: Ptychomniales, Hypnales, and Hookeriales (Shaw et al. -

Studies in the Marchantiales (Hepaticae) from Southern Africa. 6
Bothalia 24,2: 133-147 ( 1994) Studies in the Marchantiales (Hepaticae) from southern Africa. 6. The genus Asterella (Aytoniaceae: Reboulioideae) and its four local species S.M. PEROLD* Keywords: Asterella, A. bachmannii, A. marginata, A. muscicola, A. wilmsii, Aytoniaceae, Hepaticae, Marchantiales, Phragmoblepharis, Reboulioideae, section Saccatae, southern Africa ABSTRACT A taxonomic account of the genus Asterella, and four local representatives, A. muscicola, A. bachmannii, A. marginata and A. wilmsii, subgenus Phragmoblepharis, is given here. A key to the species is provided. Two specimens identified by Arnell ( ll)63) as Reboulia hemisphaerica, Collins 775 and Eyles CH 1175, are actually A. wilmsii', the presence of the genus Reboulia in southern Africa is therefore not confirmed and should be deleted from the annotated checklist by Magill & Schelpe (1979) and Plants of southern Africa: names and distribution (Arnold & De Wet 1993). UITTREKSEL n Taksonomiese verslag oor die genus Asterella, en vier van die plaaslike verteenwoordigers daarvan, A. muscicola, A. bachmannii, A. marginata cn A. wilmsii, subgenus Phragmoblepharis, word hier gegee. 'n Sleutel tot die spesies word verskaf. Twee eksemplare, Collins 775 en Eyles CH 1175, wat deur Amell (1963) as Reboulia hemisphaerica geidentifiseer is, is in werklikheid A. wilmsii', die teenwoordigheid van die genus Reboulia in Suider-Alrika is dus nie bevestig nie en dit behoort geskrap te word van die geannoteerde kontrolelys deur Magill & Schelpe (1979) en van Plants o f southern Africa: names and distribution (Arnold & EX* Wet 1993). Asterella P. Beaux, in Dictionnaire des sciences Dorsal epidermis hyaline, unistratose, cells mostly naturelles 3: 257 (1805); A. Evans: 247 (1920); Frye & thin-walled, lacking trigones, occasionally with a single L. -

Download Download
Plant Science Today (2016) 3(2): 226-236 226 http://dx.doi.org/10.14719/pst.2016.3.2.215 ISSN: 2348-1900 Plant Science Today http://horizonepublishing.com/journals/index.php/PST Research Communication Check list of Anthocerophyta and Marchantiophyta of Pakistan and Kashmir Jan Alam,1* Ibad Ali,1 Suhail Karim,1 Mazhar-ul-Islam1 and Habib Ahmad2 1Department of Botany, Hazara University, Mansehra-21300, Pakistan 2Department of Genetics, Hazara University, Mansehra-21300, Pakistan Article history Abstract Received: 16 March 2016 In the present study, a review of previously published literature regarding Accepted: 13 April 2016 Published: 22 June 2016 Anthocerophyta and Marchantiophyta of Pakistan and Kashmir has been done in order to know the diversity of these groups. Previous contributions collectively reveal 122 taxa distributed in 36 genera and 24 families. Of these © Alam et al. (2016) 118 taxa (97.52%) are belonging to the Marchantiophyta, while the rest of 4 species (3.30%) members to Anthocerophyta. Aytoniaceae is the largest family Special Section: New Frontiers in with 16 species. Genera-wise, Riccia is the largest genus with 12 species. An Cryptogamic Botany average number of species/genera is c. 3.36. A major portion of Pakistan is still un-explored especially Sindh and Balochistan province of Pakistan, and on the Section Editor basis of this study it can be said that many more taxa will be added to the list. Afroz Alam Keywords Anthocerophyta; Bryoflora; Marchantiophyta; Pakistan Publisher Horizon e-Publishing Group Alam, J., I. Ali, S. Karim, M. Islam and H. Ahmad. 2016. Check list of Corresponding Author Anthocerophyta and Marchantiophyta of Pakistan and Kashmir. -

Hypnaceaeandpossiblyrelatedfn
Hikobial3:645-665.2002 Molecularphylo窪enyOfhypnobrJ/aleanmOssesasin化rredfroma lar淫e-scaledatasetofchlOroplastlbcL,withspecialre他rencetothe HypnaceaeandpOssiblyrelatedfnmilies1 HIRoMITsuBoTA,ToMoTsuGuARIKAwA,HIRoYuKIAKIYAMA,EFRAINDELuNA,DoLoREs GoNzALEz,MASANoBuHIGucHIANDHIRoNoRIDEGucHI TsuBoTA,H、,ARIKAwA,T,AKIYAMA,H,,DELuNA,E,GoNzALEz,,.,HIGucHI,M 4 &DEGucHI,H、2002.Molecularphylogenyofhypnobryaleanmossesasinferred fiPomalarge-scaledatasetofchloroplastr6cL,withspecialreferencetotheHypnaceae andpossiblyrelatedfamiliesl3:645-665. ▲ Phylogeneticrelationshipswithinthehypnobryaleanmosses(ie,theHypnales,Leuco- dontales,andHookeriales)havebeenthefbcusofmuchattentioninrecentyears Herewepresentphylogeneticinfierencesonthislargeclade,andespeciallyonthe Hypnaceaeandpossiblyrelatedftlmilies,basedonmaximumlikelihoodanalysisof l81r6cLsequences、Oursmdycorroboratesthat(1)theHypnales(sstr.[=sensu Vittl984])andLeucodontalesareeachnotmonophyleticentities、TheHypnalesand LeucodontalestogethercompriseawellsupportedsistercladetotheHookeriales;(2) theSematophyllaceae(s」at[=sensuTsubotaetaL2000,2001a,b])andPlagiothecia‐ ceae(s・str.[=sensupresentDareeachresolvedasmonophyleticgroups,whileno particularcladeaccommodatesallmembersoftheHypnaceaeandCryphaeaceae;and (3)theHypnaceaeaswellasitstypegenusノリDlwz"川tselfwerepolyphyletioThese resultsdonotconcurwiththesystemsofVitt(1984)andBuckandVitt(1986),who suggestedthatthegroupswithasinglecostawouldhavedivergedfiFomthehypnalean ancestoratanearlyevolutionarystage,fbllowedbythegroupswithadoublecosta (seealsoTsubotaetall999;Bucketal2000)OurresultsfiPomlikelihoodanalyses -

Part 2 – Fruticose Species
Appendix 5.2-1 Vegetation Technical Appendix APPENDIX 5.2‐1 Vegetation Technical Appendix Contents Section Page Ecological Land Classification ............................................................................................................ A5.2‐1‐1 Geodatabase Development .............................................................................................. A5.2‐1‐1 Vegetation Community Mapping ..................................................................................... A5.2‐1‐1 Quality Assurance and Quality Control ............................................................................ A5.2‐1‐3 Limitations of Ecological Land Classification .................................................................... A5.2‐1‐3 Field Data Collection ......................................................................................................... A5.2‐1‐3 Supplementary Results ..................................................................................................... A5.2‐1‐4 Rare Vegetation Species and Rare Ecological Communities ........................................................... A5.2‐1‐10 Supplementary Desktop Results ..................................................................................... A5.2‐1‐10 Field Methods ................................................................................................................. A5.2‐1‐16 Supplementary Results ................................................................................................... A5.2‐1‐17 Weed Species