Chapter 3-1 Sexuality: Sexual Strategies Janice M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Novelties in the Hornwort Flora of Croatia and Southeast Europe
cryptogamie Bryologie 2019 ● 40 ● 22 DIRECTEUR DE LA PUBLICATION : Bruno David, Président du Muséum national d’Histoire naturelle RÉDACTEURS EN CHEF / EDITORS-IN-CHIEF : Denis LAMY ASSISTANTS DE RÉDACTION / ASSISTANT EDITORS : Marianne SALAÜN ([email protected]) MISE EN PAGE / PAGE LAYOUT : Marianne SALAÜN RÉDACTEURS ASSOCIÉS / ASSOCIATE EDITORS Biologie moléculaire et phylogénie / Molecular biology and phylogeny Bernard GOFFINET Department of Ecology and Evolutionary Biology, University of Connecticut (United States) Mousses d’Europe / European mosses Isabel DRAPER Centro de Investigación en Biodiversidad y Cambio Global (CIBC-UAM), Universidad Autónoma de Madrid (Spain) Francisco LARA GARCÍA Centro de Investigación en Biodiversidad y Cambio Global (CIBC-UAM), Universidad Autónoma de Madrid (Spain) Mousses d’Afrique et d’Antarctique / African and Antarctic mosses Rysiek OCHYRA Laboratory of Bryology, Institute of Botany, Polish Academy of Sciences, Krakow (Pologne) Bryophytes d’Asie / Asian bryophytes Rui-Liang ZHU School of Life Science, East China Normal University, Shanghai (China) Bioindication / Biomonitoring Franck-Olivier DENAYER Faculté des Sciences Pharmaceutiques et Biologiques de Lille, Laboratoire de Botanique et de Cryptogamie, Lille (France) Écologie des bryophytes / Ecology of bryophyte Nagore GARCÍA MEDINA Department of Biology (Botany), and Centro de Investigación en Biodiversidad y Cambio Global (CIBC-UAM), Universidad Autónoma de Madrid (Spain) COUVERTURE / COVER : Extraits d’éléments de la Figure 2 / Extracts of -

The Origin and Early Evolution of Plants on Land
review article The origin and early evolution of plants on land Paul Kenrick & Peter R. Crane . The origin and early evolution of land plants in the mid-Palaeozoic era, between about 480 and 360 million years ago, was an important event in the history of life, with far-reaching consequences for the evolution of terrestrial organisms and global environments. A recent surge of interest, catalysed by palaeobotanical discoveries and advances in the systematics of living plants, provides a revised perspective on the evolution of early land plants and suggests new directions for future research. The origin and early diversification of land plants marks an interval Eoembryophytic (mid-Ordovician [early Llanvirn: ϳ476 Myr] to of unparalleled innovation in the history of plant life. From a simple Early Silurian [late Llandovery: ϳ432 Myr])3. Spore tetrads (com- plant body consisting of only a few cells, land plants (liverworts, prising four membrane-bound spores; Fig. 2d) appear over a broad hornworts, mosses and vascular plants) evolved an elaborate two- geographic area in the mid-Ordovician and provide the first good phase life cycle and an extraordinary array of complex organs and evidence of land plants3,26,29. The combination of a decay-resistant tissue systems. Specialized sexual organs (gametangia), stems with wall (implying the presence of sporopollenin) and tetrahedral an intricate fluid transport mechanism (vascular tissue), structural configuration (implying haploid meiotic products) is diagnostic tissues (such as wood), epidermal structures for respiratory gas of land plants. The precise relationships of the spore producers exchange (stomates), leaves and roots of various kinds, diverse within land plants are controversial, but evidence of tetrads and spore-bearing organs (sporangia), seeds and the tree habit had all other spore types (such as dyads) in Late Silurian and Devonian evolved by the end of the Devonian period. -

Phytotaxa, a Synthesis of Hornwort Diversity
Phytotaxa 9: 150–166 (2010) ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ Article PHYTOTAXA Copyright © 2010 • Magnolia Press ISSN 1179-3163 (online edition) A synthesis of hornwort diversity: Patterns, causes and future work JUAN CARLOS VILLARREAL1 , D. CHRISTINE CARGILL2 , ANDERS HAGBORG3 , LARS SÖDERSTRÖM4 & KAREN SUE RENZAGLIA5 1Department of Ecology and Evolutionary Biology, University of Connecticut, 75 North Eagleville Road, Storrs, CT 06269; [email protected] 2Centre for Plant Biodiversity Research, Australian National Herbarium, Australian National Botanic Gardens, GPO Box 1777, Canberra. ACT 2601, Australia; [email protected] 3Department of Botany, The Field Museum, 1400 South Lake Shore Drive, Chicago, IL 60605-2496; [email protected] 4Department of Biology, Norwegian University of Science and Technology, N-7491 Trondheim, Norway; [email protected] 5Department of Plant Biology, Southern Illinois University, Carbondale, IL 62901; [email protected] Abstract Hornworts are the least species-rich bryophyte group, with around 200–250 species worldwide. Despite their low species numbers, hornworts represent a key group for understanding the evolution of plant form because the best–sampled current phylogenies place them as sister to the tracheophytes. Despite their low taxonomic diversity, the group has not been monographed worldwide. There are few well-documented hornwort floras for temperate or tropical areas. Moreover, no species level phylogenies or population studies are available for hornworts. Here we aim at filling some important gaps in hornwort biology and biodiversity. We provide estimates of hornwort species richness worldwide, identifying centers of diversity. We also present two examples of the impact of recent work in elucidating the composition and circumscription of the genera Megaceros and Nothoceros. -
Species Summary Table
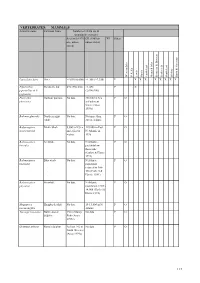
VERTEBRATES: MAMMALS Scientific name Common Name Number of 10 km sqs & (population estimate) Scotland (1970 GB (1960 on WI Status on - unless unless stated) stated) Western Isles St Kilda Lewis Harris North Uist Monach Isles Berneray & Boreray Benbecula South Uist Eriskay Barra & Vatersay Lutra lutra lutra Otter >1,050 (6,600) >1,308 (>7,350) P X X X X X X X X Pipistrellus Pipistrelle bat 492 (550,000) >1,438 P X pipisterllus & P. (2,000,000) pygmaeus Phocoena Harbour porpoise No data 350,000 in Sea P O phocoena and adjacent waters (Anon 1999a) Balaena glacialis Northern right No data Not more than P O whale 300 in Atlantic Balaenoptera Minke whale 8,500 in N Sea 110,000 in East P O acutorostrata and adjacent N. Atlantic in waters 1995 Balaenoptera Sei whale No data N Atlantic - P O borealis probably low thousands (Corbet & Harris 1991) Balaenoptera Blue whale No data N Atlantic P O musculus population reduced to 300- 500 (Corbett & Harris 1991) Balaenoptera Fin whale No data N Atlantic P O physalus population 9,000 - 14,000 (Corbet & Harris 1991) Megaptera Humpback whale No data 10-15,000 in N P O novaeangilea Atlantic Tursiops truncatus Bottle-nosed 130 in Moray No data P O dolphin Firth (Anon 1999a) Grampus griseus Risso's dolphin At least 142 in No data P O North Minches (Anon 1999a) 113 VERTEBRATES: MAMMALS Scientific name Common Name Number of 10 km sqs & (population estimate) Scotland (1970 GB (1960 on WI Status on - unless unless stated) stated) Western Isles St Kilda Lewis Harris North Uist Monach Isles Berneray & Boreray Benbecula -

And Liverworts (Marchantiophyta) of the Zackenberg Valley, Northeast Greenland
Lindbergia 37: 66–84, 2014 ISSN 2001-5909 Accepted 17 October 2014 Mosses (Bryophyta) and liverworts (Marchantiophyta) of the Zackenberg valley, northeast Greenland Kristian Hassel, Harald Zechmeister and Tommy Prestø K. Hassel ([email protected]) and T. Prestø, The Natural History Dept, NTNU University Museum, Norwegian Univ. of Science and Technology, NO-7491 Trondheim, Norway. – H. Zechmeister, Dept of Botany and Biodiversity Research, Rennweg 14, AT-1030 Vienna, Austria. The moss and liverwort flora of Zackenberg valley in the Northeast Greenland National Park has been studied based on field investigations and literature survey. Altogether 212 taxa are recorded in the area� with 43 liverworts and 169 mosses. Five taxa are reported as new to Greenland Lophochaete fryei (Perss.) R.M. Schust.� Sphagnum orientale �.I. Savicz� Orthothecium lapponicum (Schimp.) C. �artm.� Pohlia vexans (�impr.) �. �indb. and Tortella alpicola Dixon. Additionally four taxa are reported as new to east Greenland; Grimmia plagiopodia �edw.� Riccardia latifrons (�indb.) �indb. Sphagnum olafii Flatberg and Tritomaria exsectiformis (Breidl.) Schiffner ex �oeske. The bryophyte flora of the Zackenberg valley is characterised by pioneer species adapted to disturbance by frost and wind� but also more stable communities exist especially at the lower part of the valley with wet to moist tundra. The Zackenberg valley bryophyte flora shows higher similarity with the flora on Svalbard (�1%) compared with Ellesmere Island (67% and 60% for liverworts and mosses� respectively). -

A Revised Red List of Bryophytes in Britain
ConservationNews Revised Red List distinguished from Extinct. This Red List uses Extinct in the Wild (EW) – a taxon is Extinct version 3.1 of the categories and criteria (IUCN, in the Wild when it is known to survive only in A revised Red List of 2001), along with guidelines produced to assist cultivation or as a naturalized population well with their interpretation and use (IUCN, 2006, outside the past range. There are no taxa in this 2008), further guidelines for using the system category in the British bryophyte flora. bryophytes in Britain at a regional level (IUCN, 2003), and specific Regionally Extinct (RE) – a taxon is regarded guidelines for applying the system to bryophytes as Regionally Extinct in Britain if there are no (Hallingbäck et al., 1995). post-1979 records and all known localities have Conservation OfficerNick Hodgetts presents the latest revised Red List for How these categories and criteria have been been visited and surveyed without success, or interpreted and applied to the British bryophyte if colonies recorded post-1979 are known to bryophytes in Britain. Dumortiera hirsuta in north Cornwall. Ian Atherton flora is summarized below, but anyone interested have disappeared. It should be appreciated that in looking into them in more depth should regional ‘extinction’ for bryophytes is sometimes he first published Red List of et al. (2001) and Preston (2010), varieties and consult the original IUCN documents, which less final than for other, more conspicuous bryophytes in Britain was produced subspecies have been disregarded. are available on the IUCN website (www. organisms. This may be because bryophytes are in 2001 as part of a Red Data Book 1980 has been chosen as the cut-off year to iucnredlist.org/technical-documents/categories- easily overlooked, or because their very efficient for bryophytes (Church et al., 2001). -

Number of Living Species in Australia and the World
Numbers of Living Species in Australia and the World 2nd edition Arthur D. Chapman Australian Biodiversity Information Services australia’s nature Toowoomba, Australia there is more still to be discovered… Report for the Australian Biological Resources Study Canberra, Australia September 2009 CONTENTS Foreword 1 Insecta (insects) 23 Plants 43 Viruses 59 Arachnida Magnoliophyta (flowering plants) 43 Protoctista (mainly Introduction 2 (spiders, scorpions, etc) 26 Gymnosperms (Coniferophyta, Protozoa—others included Executive Summary 6 Pycnogonida (sea spiders) 28 Cycadophyta, Gnetophyta under fungi, algae, Myriapoda and Ginkgophyta) 45 Chromista, etc) 60 Detailed discussion by Group 12 (millipedes, centipedes) 29 Ferns and Allies 46 Chordates 13 Acknowledgements 63 Crustacea (crabs, lobsters, etc) 31 Bryophyta Mammalia (mammals) 13 Onychophora (velvet worms) 32 (mosses, liverworts, hornworts) 47 References 66 Aves (birds) 14 Hexapoda (proturans, springtails) 33 Plant Algae (including green Reptilia (reptiles) 15 Mollusca (molluscs, shellfish) 34 algae, red algae, glaucophytes) 49 Amphibia (frogs, etc) 16 Annelida (segmented worms) 35 Fungi 51 Pisces (fishes including Nematoda Fungi (excluding taxa Chondrichthyes and (nematodes, roundworms) 36 treated under Chromista Osteichthyes) 17 and Protoctista) 51 Acanthocephala Agnatha (hagfish, (thorny-headed worms) 37 Lichen-forming fungi 53 lampreys, slime eels) 18 Platyhelminthes (flat worms) 38 Others 54 Cephalochordata (lancelets) 19 Cnidaria (jellyfish, Prokaryota (Bacteria Tunicata or Urochordata sea anenomes, corals) 39 [Monera] of previous report) 54 (sea squirts, doliolids, salps) 20 Porifera (sponges) 40 Cyanophyta (Cyanobacteria) 55 Invertebrates 21 Other Invertebrates 41 Chromista (including some Hemichordata (hemichordates) 21 species previously included Echinodermata (starfish, under either algae or fungi) 56 sea cucumbers, etc) 22 FOREWORD In Australia and around the world, biodiversity is under huge Harnessing core science and knowledge bases, like and growing pressure. -

Biodiverse Master
Montane, Heath and Bog Habitats MONTANE, HEATH AND BOG HABITATS CONTENTS Montane, heath and bog introduction . 66 Opportunities for action in the Cairngorms . 66 The main montane, heath and bog biodiversity issues . 68 Main threats to UK montane, heath and bog Priority species in the Cairngorms . 72 UK Priority species and Locally important species accounts . 73 Cairngorms montane, heath and bog habitat accounts: • Montane . 84 • Upland heath . 87 • Blanket bog . 97 • Raised bog . 99 ‘Key’ Cairngorms montane, heath and bog species . 100 65 The Cairngorms Local Biodiversity Action Plan MONTANE, HEATH AND BOG INTRODUCTION Around one third of the Cairngorms Partnership area is over 600-650m above sea level (above the natural woodland line, although this is variable from place to place.). This comprises the largest and highest area of montane habitat in Britain, much of which is in a relatively pristine condition. It contains the main summits and plateaux with their associated corries, rocky cliffs, crags, boulder fields, scree slopes and the higher parts of some glens and passes. The vegeta- tion is influenced by factors such as exposure, snow cover and soil type. The main zone is considered to be one of the most spectacular mountain areas in Britain and is recognised nationally and internationally for the quality of its geology, geomorphology and topographic features, and associated soils and biodiversity. c14.5% of the Cairngorms Partnership area (75,000ha) is land above 600m asl. Upland heathland is the most extensive habitat type in the Cairngorms Partnership area, covering c41% of the area, frequently in mosaics with blanket bog. -

Anthocerotophyta
Glime, J. M. 2017. Anthocerotophyta. Chapt. 2-8. In: Glime, J. M. Bryophyte Ecology. Volume 1. Physiological Ecology. Ebook 2-8-1 sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 5 June 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 2-8 ANTHOCEROTOPHYTA TABLE OF CONTENTS Anthocerotophyta ......................................................................................................................................... 2-8-2 Summary .................................................................................................................................................... 2-8-10 Acknowledgments ...................................................................................................................................... 2-8-10 Literature Cited .......................................................................................................................................... 2-8-10 2-8-2 Chapter 2-8: Anthocerotophyta CHAPTER 2-8 ANTHOCEROTOPHYTA Figure 1. Notothylas orbicularis thallus with involucres. Photo by Michael Lüth, with permission. Anthocerotophyta These plants, once placed among the bryophytes in the families. The second class is Leiosporocerotopsida, a Anthocerotae, now generally placed in the phylum class with one order, one family, and one genus. The genus Anthocerotophyta (hornworts, Figure 1), seem more Leiosporoceros differs from members of the class distantly related, and genetic evidence may even present -

Flora of New Zealand Mosses
FLORA OF NEW ZEALAND MOSSES BRACHYTHECIACEAE A.J. FIFE Fascicle 46 – JUNE 2020 © Landcare Research New Zealand Limited 2020. Unless indicated otherwise for specific items, this copyright work is licensed under the Creative Commons Attribution 4.0 International licence Attribution if redistributing to the public without adaptation: "Source: Manaaki Whenua – Landcare Research" Attribution if making an adaptation or derivative work: "Sourced from Manaaki Whenua – Landcare Research" See Image Information for copyright and licence details for images. CATALOGUING IN PUBLICATION Fife, Allan J. (Allan James), 1951- Flora of New Zealand : mosses. Fascicle 46, Brachytheciaceae / Allan J. Fife. -- Lincoln, N.Z. : Manaaki Whenua Press, 2020. 1 online resource ISBN 978-0-947525-65-1 (pdf) ISBN 978-0-478-34747-0 (set) 1. Mosses -- New Zealand -- Identification. I. Title. II. Manaaki Whenua-Landcare Research New Zealand Ltd. UDC 582.345.16(931) DC 588.20993 DOI: 10.7931/w15y-gz43 This work should be cited as: Fife, A.J. 2020: Brachytheciaceae. In: Smissen, R.; Wilton, A.D. Flora of New Zealand – Mosses. Fascicle 46. Manaaki Whenua Press, Lincoln. http://dx.doi.org/10.7931/w15y-gz43 Date submitted: 9 May 2019 ; Date accepted: 15 Aug 2019 Cover image: Eurhynchium asperipes, habit with capsule, moist. Drawn by Rebecca Wagstaff from A.J. Fife 6828, CHR 449024. Contents Introduction..............................................................................................................................................1 Typification...............................................................................................................................................1 -

North American H&A Names
A very tentative and preliminary list of North American liverworts and hornworts, doubtless containing errors and omissions, but forming a basis for updating the spreadsheet of recognized genera and numbers of species, November 2010. Liverworts Blasiales Blasiaceae Blasia L. Blasia pusilla L. Fossombroniales Calyculariaceae Calycularia Mitt. Calycularia crispula Mitt. Calycularia laxa Lindb. & Arnell Fossombroniaceae Fossombronia Raddi Fossombronia alaskana Steere & Inoue Fossombronia brasiliensis Steph. Fossombronia cristula Austin Fossombronia foveolata Lindb. Fossombronia hispidissima Steph. Fossombronia lamellata Steph. Fossombronia macounii Austin Fossombronia marshii J. R. Bray & Stotler Fossombronia pusilla (L.) Dumort. Fossombronia longiseta (Austin) Austin Note: Fossombronia longiseta was based on a mixture of material belonging to three different species of Fossombronia; Schuster (1992a p. 395) lectotypified F. longiseta with the specimen of Austin, Hepaticae Boreali-Americani 118 at H. An SEM of one spore from this specimen was previously published by Scott and Pike (1988 fig. 19) and it is clearly F. pusilla. It is not at all clear why Doyle and Stotler (2006) apply the name to F. hispidissima. Fossombronia texana Lindb. Fossombronia wondraczekii (Corda) Dumort. Fossombronia zygospora R.M. Schust. Petalophyllum Nees & Gottsche ex Lehm. Petalophyllum ralfsii (Wilson) Nees & Gottsche ex Lehm. Moerckiaceae Moerckia Gottsche Moerckia blyttii (Moerch) Brockm. Moerckia hibernica (Hook.) Gottsche Pallaviciniaceae Pallavicinia A. Gray, nom. cons. Pallavicinia lyellii (Hook.) Carruth. Pelliaceae Pellia Raddi, nom. cons. Pellia appalachiana R.M. Schust. (pro hybr.) Pellia endiviifolia (Dicks.) Dumort. Pellia endiviifolia (Dicks.) Dumort. ssp. alpicola R.M. Schust. Pellia endiviifolia (Dicks.) Dumort. ssp. endiviifolia Pellia epiphylla (L.) Corda Pellia megaspora R.M. Schust. Pellia neesiana (Gottsche) Limpr. Pellia neesiana (Gottsche) Limpr. -

Part 2 – Fruticose Species
Appendix 5.2-1 Vegetation Technical Appendix APPENDIX 5.2‐1 Vegetation Technical Appendix Contents Section Page Ecological Land Classification ............................................................................................................ A5.2‐1‐1 Geodatabase Development .............................................................................................. A5.2‐1‐1 Vegetation Community Mapping ..................................................................................... A5.2‐1‐1 Quality Assurance and Quality Control ............................................................................ A5.2‐1‐3 Limitations of Ecological Land Classification .................................................................... A5.2‐1‐3 Field Data Collection ......................................................................................................... A5.2‐1‐3 Supplementary Results ..................................................................................................... A5.2‐1‐4 Rare Vegetation Species and Rare Ecological Communities ........................................................... A5.2‐1‐10 Supplementary Desktop Results ..................................................................................... A5.2‐1‐10 Field Methods ................................................................................................................. A5.2‐1‐16 Supplementary Results ................................................................................................... A5.2‐1‐17 Weed Species