Impact of Persistent Left Superior Vena Cava Detection in a Normal Neonatal Population Through Echocardiography
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abnormal Embryonic Lymphatic Vessel Development in Tie1 Hypomorphic Mice Xianghu Qu, Kevin Tompkins, Lorene E
© 2014. Published by The Company of Biologists Ltd | Development (2014) 141, 1417 doi:10.1242/dev.108969 CORRECTION Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice Xianghu Qu, Kevin Tompkins, Lorene E. Batts, Mira Puri and H. Scott Baldwin There was an error published in Development 137, 1285-1295. Author name H. Scott Baldwin was incomplete. The correct author list appears above. The authors apologise to readers for this mistake. 1417 RESEARCH ARTICLE 1285 Development 137, 1285-1295 (2010) doi:10.1242/dev.043380 © 2010. Published by The Company of Biologists Ltd Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice Xianghu Qu1, Kevin Tompkins1, Lorene E. Batts1, Mira Puri2 and Scott Baldwin1,3,* SUMMARY Tie1 is an endothelial receptor tyrosine kinase that is essential for development and maintenance of the vascular system; however, the role of Tie1 in development of the lymphatic vasculature is unknown. To address this question, we first documented that Tie1 is expressed at the earliest stages of lymphangiogenesis in Prox1-positive venous lymphatic endothelial cell (LEC) progenitors. LEC Tie1 expression is maintained throughout embryonic development and persists in postnatal mice. We then generated two lines of Tie1 mutant mice: a hypomorphic allele, which has reduced expression of Tie1, and a conditional allele. Reduction of Tie1 levels resulted in abnormal lymphatic patterning and in dilated and disorganized lymphatic vessels in all tissues examined and in impaired lymphatic drainage in embryonic skin. Homozygous hypomorphic mice also exhibited abnormally dilated jugular lymphatic vessels due to increased production of Prox1-positive LECs during initial lymphangiogenesis, indicating that Tie1 is required for the early stages of normal lymphangiogenesis. -

The Evolving Cardiac Lymphatic Vasculature in Development, Repair and Regeneration
REVIEWS The evolving cardiac lymphatic vasculature in development, repair and regeneration Konstantinos Klaourakis 1,2, Joaquim M. Vieira 1,2,3 ✉ and Paul R. Riley 1,2,3 ✉ Abstract | The lymphatic vasculature has an essential role in maintaining normal fluid balance in tissues and modulating the inflammatory response to injury or pathogens. Disruption of normal development or function of lymphatic vessels can have severe consequences. In the heart, reduced lymphatic function can lead to myocardial oedema and persistent inflammation. Macrophages, which are phagocytic cells of the innate immune system, contribute to cardiac development and to fibrotic repair and regeneration of cardiac tissue after myocardial infarction. In this Review, we discuss the cardiac lymphatic vasculature with a focus on developments over the past 5 years arising from the study of mammalian and zebrafish model organisms. In addition, we examine the interplay between the cardiac lymphatics and macrophages during fibrotic repair and regeneration after myocardial infarction. Finally, we discuss the therapeutic potential of targeting the cardiac lymphatic network to regulate immune cell content and alleviate inflammation in patients with ischaemic heart disease. The circulatory system of vertebrates is composed of two after MI. In this Review, we summarize the current complementary vasculatures, the blood and lymphatic knowledge on the development, structure and function vascular systems1. The blood vasculature is a closed sys- of the cardiac lymphatic vasculature, with an emphasis tem responsible for transporting gases, fluids, nutrients, on breakthroughs over the past 5 years in the study of metabolites and cells to the tissues2. This extravasation of cardiac lymphatic heterogeneity in mice and zebrafish. -
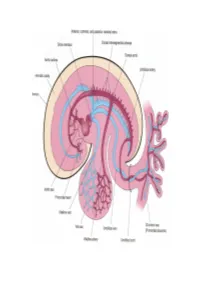
Development of HEART 4-VEINS
Development of brachiocephalic veins 1. Right brachiocephalic vein is formed by cranial part of right anterior cardinal vein and 2. Left brachiocephalic is formed by cranial part of left anterior cardinal vein and the interant.cardinal anastomosis. Development of superior vena cava 1. The part up to the opening of vena azygos develops from caudal part of right ant.cardinal vein and 2. The part below the opening (intrapericardial part) is formed by the right common cardinal vein. Development of azygos and hemiazygos veins A. 1. Vena azygos develops from right azygos line vein and 2. The arch of vena azygos is formed by the cranial end of right postcardinal vein. B. Hemiazygos veins are formed by the left azygos line vein. Development of Inferior vena cava Inferior vena cava is formed, from below upwards by: 1. Begins by the union of the two common iliac veins (postcardinal veins), 2. Right supracardinal, 3. Right supra-subcardinal anastomosis, 4. Right subcardinal, 5. New formation (hepatic segment) and 6. Hepatocardiac channel (terminal part of right vitelline vein). Congenital anomalies • Double inferior vena cava • Absence • Left SVC • Double SVC DEVELOPMENT OF PORTAL VEIN 1. The portal vein is formed behind the neck of pancreas by the union of superior mesentric and splenic vein to the left vitelline vein. 2. The part of the portal vein which is behind the Ist part of duodenum is formed by middle dorsal transverse anastomosis. 3. Part of portal vein which is in the free margin of lesser omentum is formed by cranial or distal part of right vitelline vein. -

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -
Development and Teratology of Cardiovascular and Lymphatic Systems
Development and teratology of cardiovascular and lymphatic systems Repetition: Muscle tissue Beginning of the cardiovascular system development – the 3rd week: Hemangiogenesis (day 15 – 16) – blood islets (insulae sanguinae) in extraembryonic mesoderm and splanchnic mesenchyme of embryo Clusters of mesenchyme cells (angiogenic cells) differentiate into: - angioblasts endothelium (at the periphery of blood islets) - hemoblasts primitive erythrocytes (in the center of blood islets) Clusters of angiogenic cells form a "horseshoe-shaped" space between somatic and splanchnic layer of mesoderm = pericardial cavity. Two endothelial tubes arrise in splanchnic mesoderm. The ventral portion of these tubes forms the cardiogenic area with two heart tubes, while the lateral portions form the dorsal aortae. Germ disc: prosencephalon mesencephalon eye rhombencephalon heart lateral mesoderm somites small blood vessels blood islands 8,9 Spine primitive streak Initially, the cardiogenic area is located anterior to the prechordal plate and the neural plate. The growth of the central nervous system pulls the cardiogenic area and prechordal plate (buccopharyngeal membrane ventrally and caudally ( ). Cardiogenic region just cranial to the prechordal plate. The canalization of cardiogenic clusters in the splanchnic mesoderm results in the formation of the paired heart tubes. Folding of embryo and primitive gut separation from yolk sac. Fusion of the heart tubes a single heart tube is, temporarily attached to the dorsal side of the pericardial cavity by the -

6 Development of the Great Vessels and Conduction Tissue
Development of the Great Vessels and Conduc6on Tissue Development of the heart fields • h:p://php.med.unsw.edu.au/embryology/ index.php?6tle=Advanced_-_Heart_Fields ! 2 Septa6on of the Bulbus Cordis Bulbus Cordis AV Canal Ventricle Looking at a sagital sec6on of the heart early in development the bulbus cordis is con6nuous with the ventricle which is con6nuous with the atria. As the AV canal shiOs to the right the bulbus move to the right as well. Septa6on of the Bulbus Cordis A P A P The next three slides make the point via cross sec6ons that the aorta and pulmonary arteries rotate around each other. This means the septum between them changes posi6on from superior to inferior as well. Septa6on of the Bulbus Cordis P A A P Septa6on of the Bulbus Cordis P A P A Migra6on of neural crest cells Neural crest cells migrate from the 3ed, 4th and 6th pharyngeal arches to form some of the popula6on of cells forming the aor6copulmonary septum. Septa6on of the Bulbus Cordis Truncal (Conal) Swellings Bulbus Cordis The cardiac jelly in the region of the truncus and conus adds the neural crest cells and expands as truncal swellings. Septa6on of the Bulbus Cordis Aorticopulmonary septum These swellings grow toward each other to meet and form the septum between the aorta and pulmonary artery. Aorta Pulmonary Artery Septa6on of the Bulbus Cordis Anterior 1 2 3 1 2 3 The aor6copulmonary septum then rotates as it moves inferiorly. However, the exact mechanism for that rota6on remains unclear. Septa6on of the Bulbus Cordis Aorta Pulmonary Artery Conal Ridges IV Foramen Membranous Muscular IV Endocarial Septum Interventricular Cushion Septum However, the aor6copulmonary septum must form properly for the IV septum to be completed. -

Dosage-Sensitive Requirement for Mouse Dll4 in Artery Development
Downloaded from genesdev.cshlp.org on September 26, 2021 - Published by Cold Spring Harbor Laboratory Press RESEARCH COMMUNICATION Dosage-sensitive requirement 2000), which were also observed, to a lesser degree, in Hey1/Hey2 double mutants, the putative downstream for mouse Dll4 targets of Notch signaling (Fischer et al. 2004). Here we in artery development show, in contrast, that the Dll4 ligand alone is required in a dosage-sensitive manner for normal arterial pattern- António Duarte,1,5 Masanori Hirashima,3,5,6 ing in development. Rui Benedito,1 Alexandre Trindade,1 The Dll4 gene was inactivated by targeted disruption 1 2 1 in embryonic stem (ES) cells. The targeting vector was Patrícia Diniz, Evguenia Bekman, Luís Costa, designed to replace the initiation codon and the first 2 3,4,7 Domingos Henrique, and Janet Rossant three coding exons with the -galactosidase (lacZ) re- porter gene (Fig. 1A). Electroporation of R1 ES cells with 1CIISA, Faculdade de Medicina Veterina´ria, 1300-0-477 the targeting vector followed by G418 selection resulted Lisboa, Portugal; 2Instituto de Medicina Molecular, Faculdade in the derivation of four correctly gene-targeted ES cell de Medicina, 1649-9-028 Lisboa, Portugal; 3Samuel Lunenfeld lines. Chimeric mice were generated by aggregation be- Research Institute, Mount Sinai Hospital, Toronto, Ontario, tween Dll4+/− ES cells and ICR host embryos. When Canada M5G1X5; 4Department of Molecular and Medical crossed with wild-type ICR females, five male chimeras Genetics, University of Toronto, Toronto, Ontario, produced F1 agouti pups at 100% frequency. Genotyping Canada M5S 1A8 of F1 agouti mice by Southern blotting or polymerase +/− Involvement of the Notch signaling pathway in vascular chain reaction (PCR; Fig. -

LAC.12 Embryology 2019-2020 Dr.Mahdi Alheety
LAC.12 Embryology 2019-2020 Dr.Mahdi ALheety Cardiovascular System Establishment of the Cardiogenic Field The vascular system appears in the middle of the third week, when the embryo is no longer able to satisfy its nutritional requirements by diffusion alone. Progenitor heart cells lie in the epiblast, immediately adjacent to the cranial end of the primitive streak. From there, they migrate through the streak and into the splanchnic layer of lateral plate mesoderm where they form a horseshoe-shaped cluster of cells called the primary heart field (PHF) cranial to the neural folds. As the progenitor heart cells migrate and form the PHF during days 16 to18, they are specified on both sides from lateral to medial to become the atria, left ventricle, and most of the right ventricle. Patterning of these cells occurs at the same time that laterality (left-right sidedness) is being established for the entire embryo and this process and the signaling pathway it is dependent upon is essential for normal heart development. The remainder of the heart, including part of the right ventricle and outflow tract (conus cordis and truncus arteriosus), is derived from the secondary heart field (SHF). This field of cells appears slightly later (days 20 to 21) than those in the PHF, resides in splanchnic mesoderm ventral to the posterior pharynx, and is responsible for lengthening the outflow tract. Cells in the SHF also exhibit laterality, such that those on the right side contribute to the left of the outflow tract region and those on the left contribute to the right. -

Anomalous Right Superior Vena Cava in an Asymptomatic Patient
Open Access Case Report DOI: 10.7759/cureus.8265 Anomalous Right Superior Vena Cava in an Asymptomatic Patient Hardik Bhatt 1 , Megan McGreevy 2 , Charles J. Chung 3 , Sharma Kattel 4 1. Internal Medicine, University at Buffalo, Buffalo, USA 2. Pediatric Cardiology, Oishei Children's Hospital, Buffalo, USA 3. Radiology, State University of New York, Buffalo, USA 4. Cardiology, University at Buffalo, Buffalo, USA Corresponding author: Hardik Bhatt, [email protected] Abstract Congenital superior vena cava (SVC) anomalies are not uncommon. However, an absence of a left SVC and an anomalous right SVC without additional congenital heart defects is very rare. We present a 38-year-old male with an 'anomalous SVC' that was found to be descending anterior to the pleural space and draining into the inferior vena cava (IVC) at the level of the right atrium. This was associated with an anomalous right upper and lower pulmonary vein draining into this anomalous SVC. To our knowledge, this combination of congenital anomalies has not been previously described in the medical literature. Categories: Cardiology, Internal Medicine Keywords: anomalous svc, adult congenital heart disease, absent right superior vena cava, azygos vein, congenital heart disease Introduction Congenital systemic venous anomalies have been well documented in the medical literature. Specifically, anomalies of the superior vena cava (SVC) have been commonly found in asymptomatic patients. The most common anomaly of the SVC is the persistence of a left-sided SVC, seen in about 10% of patients with congenital heart disease and 0.5%-2% of the general population [1]. Additionally, the presence of a left- sided SVC in the absence of a right-sided SVC has been found to be in 0.09%-0.13% of the population [2]. -

Lymphatic Vasculature Development
R E V I E W S LYMPHATIC VASCULATURE DEVELOPMENT Guillermo Oliver Although the process of blood vasculature formation has been well documented, little is known about lymphatic vasculature development, despite its importance in normal and pathological conditions. The lack of specific lymphatic markers has hampered progress in this field. However, the recent identification of genes that participate in the formation of the lymphatic vasculature denotes the beginning of a new era in which better diagnoses and therapeutic treatment(s) of lymphatic disorders could become a reachable goal. LYMPHANGIOGENESIS Lymphatic vessels were first described in the seventeenth agent, afferent lymphatic vessels are the route through The growth of lymphatic vessels. century by G.Aselli1. However, until a few years ago, the which, after antigen uptake, dendritic cells migrate to lymphatic system was not considered an interesting topic the lymph nodes and lymphoid organs. Recent studies LYMPHOEDEMA among most scientists. Despite the importance of the indicate that spindle cells and cells lining the irregular Occlusion of lymphatic drainage followed by the lymphatic system and the belief that metastatic tumour spaces in Kaposi’s sarcoma have a lymphatic endothelial abnormal accumulation of cells are disseminated through the lymphatic vasculature, origin9,10. An increased understanding of normal interstitial fluid and swelling modern anatomy textbooks generally include only a lymphatic development should allow us to address in the affected body part. minimal description of the development of this system. pathological lymphatic conditions that lead to inflam- LYMPHATIC METASTASIS Interest in basic lymphatic research has radically mation, autoimmunity and cancer, and to improve the Invasion of detaching primary increased in the past few years,in part, thanks to the iden- clinical treatment of primary and secondary forms of tumour cells through the tification of lymphatic-specific markers and growth lymphoedema. -

The Development of the Vascular System in Quail Embryos: a Combination of Microvascular Corrosion Casts and Immunohistochemical Identification
Scanning Microscopy Volume 5 Number 4 Article 17 10-16-1991 The Development of the Vascular System in Quail Embryos: A Combination of Microvascular Corrosion Casts and Immunohistochemical Identification M. C. DeRuiter University of Leiden B. Hogers University of Leiden R. E. Poelmann University of Leiden L. Vanlperen University of Leiden A. C. Gittenberger-de Groot University of Leiden Follow this and additional works at: https://digitalcommons.usu.edu/microscopy Part of the Biology Commons Recommended Citation DeRuiter, M. C.; Hogers, B.; Poelmann, R. E.; Vanlperen, L.; and Gittenberger-de Groot, A. C. (1991) "The Development of the Vascular System in Quail Embryos: A Combination of Microvascular Corrosion Casts and Immunohistochemical Identification," Scanning Microscopy: Vol. 5 : No. 4 , Article 17. Available at: https://digitalcommons.usu.edu/microscopy/vol5/iss4/17 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Scanning Microscopy by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Scanning Microscopy, Vol. 5, No. 4, 1991 (Pages 1081-1090) 0891- 7035/91$3.00+. 00 Scanning Microscopy International, Chicago (AMF O'Hare), IL 60666 USA THE DEVELOPMENT OF THE VASCULAR SYSTEM IN QUAIL EMBRYOS : ' A COMBINATION OF MICROVASCULAR CORROSION CASTS AND IMMUNOHISTOCHEMICAL IDENTIFICATION M.C.DeRuiter , B.Hogers , R.E.Poelmann L.Vanlperen, A.C .Gittenberger-de Groot* Dept. of Anatomy and Embryology, University of Leiden. The Netherlands. (Received for publication June 11, 1991, and in revised form October 16, 1991) Abstract Introduction Although vascular casts, obtained by injection with methacrylates, are frequently used The last decade has seen more frequent application of the microcorrosion casting to investigate the adult vascu_lar system, l1ttl_e data are available for embryonic stages technique in combination with scanning electron . -

A Crucial Role of Caldesmon in Vascular Development in Vivo
Cardiovascular Research (2009) 81, 362–369 doi:10.1093/cvr/cvn294 A crucial role of caldesmon in vascular development in vivo Ping-Pin Zheng1, Lies-Anne Severijnen2, Marcel van der Weiden1, Rob Willemsen2†, and Johan M. Kros1*† 1Department of Pathology, Erasmus Medical Center, JNI Room 230-c, Dr Molewaterplein 50, PO Box 1738, 3000 DR Rotterdam, The Netherlands; and 2Department of Clinical Genetics, Erasmus Medical Center, Rotterdam, The Netherlands Downloaded from https://academic.oup.com/cardiovascres/article/81/2/362/286189 by guest on 24 September 2021 Received 24 July 2008; revised 28 October 2008; accepted 29 October 2008; online publish-ahead-of-print 3 November 2008 Time for primary review: 13 days KEYWORDS Aims We explored the in vivo effects of knockdown of caldesmon on vascular development in zebrafish. Caldesmon; Methods and results We investigated the effects of caldesmon knockdown on the vascular development Vascular development; in a zebrafish model with special attention for the trunk and head vessels including the aortic arches. We Zebrafish model; examined the developing fishes at various time points. The vascular abnormalities observed in the cal- Vasculogenesis; desmon morphants were morphologically and functionally characterized in detail in fixed and living Angiogenesis embryos. The knockdown of caldesmon caused serious defects in vasculogenesis and angiogenesis in zebrafish morphants, and the vascular integrity and blood circulation were concomitantly impaired. Conclusion The data provide the first functional assessment of the role of caldesmon in vascular development in vivo, indicating that this molecule plays a crucial role in vasculogenesis and angio- genesis in vivo. Interfering with caldesmon opens new therapeutic avenues for anti-angiogenesis in cancer and ischaemic cardiovascular disease.