Lineage Tracing Demonstrates the Venous Origin of the Mammalian Lymphatic Vasculature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coronary Arterial Development Is Regulated by a Dll4-Jag1-Ephrinb2 Signaling Cascade
RESEARCH ARTICLE Coronary arterial development is regulated by a Dll4-Jag1-EphrinB2 signaling cascade Stanislao Igor Travisano1,2, Vera Lucia Oliveira1,2, Bele´ n Prados1,2, Joaquim Grego-Bessa1,2, Rebeca Pin˜ eiro-Sabarı´s1,2, Vanesa Bou1,2, Manuel J Go´ mez3, Fa´ tima Sa´ nchez-Cabo3, Donal MacGrogan1,2*, Jose´ Luis de la Pompa1,2* 1Intercellular Signalling in Cardiovascular Development and Disease Laboratory, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), Madrid, Spain; 2CIBER de Enfermedades Cardiovasculares, Madrid, Spain; 3Bioinformatics Unit, Centro Nacional de Investigaciones Cardiovasculares, Madrid, Spain Abstract Coronaries are essential for myocardial growth and heart function. Notch is crucial for mouse embryonic angiogenesis, but its role in coronary development remains uncertain. We show Jag1, Dll4 and activated Notch1 receptor expression in sinus venosus (SV) endocardium. Endocardial Jag1 removal blocks SV capillary sprouting, while Dll4 inactivation stimulates excessive capillary growth, suggesting that ligand antagonism regulates coronary primary plexus formation. Later endothelial ligand removal, or forced expression of Dll4 or the glycosyltransferase Mfng, blocks coronary plexus remodeling, arterial differentiation, and perivascular cell maturation. Endocardial deletion of Efnb2 phenocopies the coronary arterial defects of Notch mutants. Angiogenic rescue experiments in ventricular explants, or in primary human endothelial cells, indicate that EphrinB2 is a critical effector of antagonistic Dll4 and Jag1 functions in arterial morphogenesis. Thus, coronary arterial precursors are specified in the SV prior to primary coronary plexus formation and subsequent arterial differentiation depends on a Dll4-Jag1-EphrinB2 signaling *For correspondence: [email protected] (DMG); cascade. [email protected] (JLP) Competing interests: The authors declare that no Introduction competing interests exist. -

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Abnormal Embryonic Lymphatic Vessel Development in Tie1 Hypomorphic Mice Xianghu Qu, Kevin Tompkins, Lorene E
© 2014. Published by The Company of Biologists Ltd | Development (2014) 141, 1417 doi:10.1242/dev.108969 CORRECTION Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice Xianghu Qu, Kevin Tompkins, Lorene E. Batts, Mira Puri and H. Scott Baldwin There was an error published in Development 137, 1285-1295. Author name H. Scott Baldwin was incomplete. The correct author list appears above. The authors apologise to readers for this mistake. 1417 RESEARCH ARTICLE 1285 Development 137, 1285-1295 (2010) doi:10.1242/dev.043380 © 2010. Published by The Company of Biologists Ltd Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice Xianghu Qu1, Kevin Tompkins1, Lorene E. Batts1, Mira Puri2 and Scott Baldwin1,3,* SUMMARY Tie1 is an endothelial receptor tyrosine kinase that is essential for development and maintenance of the vascular system; however, the role of Tie1 in development of the lymphatic vasculature is unknown. To address this question, we first documented that Tie1 is expressed at the earliest stages of lymphangiogenesis in Prox1-positive venous lymphatic endothelial cell (LEC) progenitors. LEC Tie1 expression is maintained throughout embryonic development and persists in postnatal mice. We then generated two lines of Tie1 mutant mice: a hypomorphic allele, which has reduced expression of Tie1, and a conditional allele. Reduction of Tie1 levels resulted in abnormal lymphatic patterning and in dilated and disorganized lymphatic vessels in all tissues examined and in impaired lymphatic drainage in embryonic skin. Homozygous hypomorphic mice also exhibited abnormally dilated jugular lymphatic vessels due to increased production of Prox1-positive LECs during initial lymphangiogenesis, indicating that Tie1 is required for the early stages of normal lymphangiogenesis. -

Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease
Journal of Cardiovascular Development and Disease Review Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease Laura A. Dyer 1 and Sandra Rugonyi 2,* 1 Department of Biology, University of Portland, Portland, OR 97203, USA; [email protected] 2 Department of Biomedical Engineering, Oregon Health & Science University, Portland, OR 97239, USA * Correspondence: [email protected] Abstract: In congenital heart disease, the presence of structural defects affects blood flow in the heart and circulation. However, because the fetal circulation bypasses the lungs, fetuses with cyanotic heart defects can survive in utero but need prompt intervention to survive after birth. Tetralogy of Fallot and persistent truncus arteriosus are two of the most significant conotruncal heart defects. In both defects, blood access to the lungs is restricted or non-existent, and babies with these critical conditions need intervention right after birth. While there are known genetic mutations that lead to these critical heart defects, early perturbations in blood flow can independently lead to critical heart defects. In this paper, we start by comparing the fetal circulation with the neonatal and adult circulation, and reviewing how altered fetal blood flow can be used as a diagnostic tool to plan interventions. We then look at known factors that lead to tetralogy of Fallot and persistent truncus arteriosus: namely early perturbations in blood flow and mutations within VEGF-related pathways. The interplay between physical and genetic factors means that any one alteration can cause significant disruptions during development and underscore our need to better understand the effects of both blood flow and flow-responsive genes. -

Extensive Vasculogenesis, Angiogenesis, and Organogenesis Precede Lethality in Mice Lacking All V Integrins
Cell, Vol. 95, 507–519, November 13, 1998, Copyright ©1998 by Cell Press Extensive Vasculogenesis, Angiogenesis, and Organogenesis Precede Lethality in Mice Lacking All ␣v Integrins Bernhard L. Bader,*‡ Helen Rayburn,* their ligands. Significant expression of ␣v integrins has Denise Crowley,* and Richard O. Hynes*† been noted, in particular, in neural crest cells (Delannet * Howard Hughes Medical Institute et al., 1994), glial cells (Hirsch et al., 1994; Milner and Center for Cancer Research ffrench-Constant, 1994), muscle (Hirsch et al., 1994; Mc- and Department of Biology Donald et al., 1995; Martin and Sanes, 1997), osteoclasts Massachusetts Institute of Technology (Va¨ a¨ na¨ nen and Horton, 1995), epithelia (␣v6; Breuss et Cambridge, Massachusetts 02139 al., 1995; Huang et al., 1996), and blood vessels during development (Brooks et al., 1994a; Drake et al., 1995; Friedlander et al., 1995, 1996) or angiogenesis in re- Summary sponse to tumors (Brooks et al., 1994a, 1994b, 1996, 1998; Varner et al., 1995). ␣v integrins have been implicated in many develop- Among the ligands for various ␣v integrins is fibro- mental processes and are therapeutic targets for inhi- nectin (FN), and results on mouse embryos lacking FN bition of angiogenesis and osteoporosis. Surprisingly, or FN receptor integrins suggest that ␣v integrins might ablation of the gene for the ␣v integrin subunit, elimi- be important receptors for FN during early development. nating all five ␣v integrins, although causing lethality, FN-null embryos fail to form notochord or somites allows considerable development and organogenesis (George et al., 1993; Georges-Labouesse et al., 1996), including, most notably, extensive vasculogenesis and whereas embryos null for either (Yang et al., 1993, 1995) angiogenesis. -

The Evolving Cardiac Lymphatic Vasculature in Development, Repair and Regeneration
REVIEWS The evolving cardiac lymphatic vasculature in development, repair and regeneration Konstantinos Klaourakis 1,2, Joaquim M. Vieira 1,2,3 ✉ and Paul R. Riley 1,2,3 ✉ Abstract | The lymphatic vasculature has an essential role in maintaining normal fluid balance in tissues and modulating the inflammatory response to injury or pathogens. Disruption of normal development or function of lymphatic vessels can have severe consequences. In the heart, reduced lymphatic function can lead to myocardial oedema and persistent inflammation. Macrophages, which are phagocytic cells of the innate immune system, contribute to cardiac development and to fibrotic repair and regeneration of cardiac tissue after myocardial infarction. In this Review, we discuss the cardiac lymphatic vasculature with a focus on developments over the past 5 years arising from the study of mammalian and zebrafish model organisms. In addition, we examine the interplay between the cardiac lymphatics and macrophages during fibrotic repair and regeneration after myocardial infarction. Finally, we discuss the therapeutic potential of targeting the cardiac lymphatic network to regulate immune cell content and alleviate inflammation in patients with ischaemic heart disease. The circulatory system of vertebrates is composed of two after MI. In this Review, we summarize the current complementary vasculatures, the blood and lymphatic knowledge on the development, structure and function vascular systems1. The blood vasculature is a closed sys- of the cardiac lymphatic vasculature, with an emphasis tem responsible for transporting gases, fluids, nutrients, on breakthroughs over the past 5 years in the study of metabolites and cells to the tissues2. This extravasation of cardiac lymphatic heterogeneity in mice and zebrafish. -
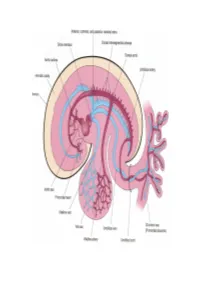
Development of HEART 4-VEINS
Development of brachiocephalic veins 1. Right brachiocephalic vein is formed by cranial part of right anterior cardinal vein and 2. Left brachiocephalic is formed by cranial part of left anterior cardinal vein and the interant.cardinal anastomosis. Development of superior vena cava 1. The part up to the opening of vena azygos develops from caudal part of right ant.cardinal vein and 2. The part below the opening (intrapericardial part) is formed by the right common cardinal vein. Development of azygos and hemiazygos veins A. 1. Vena azygos develops from right azygos line vein and 2. The arch of vena azygos is formed by the cranial end of right postcardinal vein. B. Hemiazygos veins are formed by the left azygos line vein. Development of Inferior vena cava Inferior vena cava is formed, from below upwards by: 1. Begins by the union of the two common iliac veins (postcardinal veins), 2. Right supracardinal, 3. Right supra-subcardinal anastomosis, 4. Right subcardinal, 5. New formation (hepatic segment) and 6. Hepatocardiac channel (terminal part of right vitelline vein). Congenital anomalies • Double inferior vena cava • Absence • Left SVC • Double SVC DEVELOPMENT OF PORTAL VEIN 1. The portal vein is formed behind the neck of pancreas by the union of superior mesentric and splenic vein to the left vitelline vein. 2. The part of the portal vein which is behind the Ist part of duodenum is formed by middle dorsal transverse anastomosis. 3. Part of portal vein which is in the free margin of lesser omentum is formed by cranial or distal part of right vitelline vein. -

The Reproductive System: Embryology and Human Development
28 The Reproductive System: Embryology and Human Development PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • Development involves: • Differentiation of cells • Reorganization of cells • Development can be characterized by different periods of time • Prenatal development • Embryology • Postnatal development © 2012 Pearson Education, Inc. An Overview of Development • Development can be characterized by different periods of time • Prenatal development • Conception to delivery • Involves embryology (development during the prenatal period) • Postnatal development • Development from birth to maturity © 2012 Pearson Education, Inc. Fertilization • Fertilization is the joining of two haploid cells to create a diploid cell • Function of the haploid cells • Spermatozoon • Delivers the paternal chromosomes to the ovum • Ovum • Provides the maternal chromosomes • Provides nourishment for embryonic development © 2012 Pearson Education, Inc. Fertilization • Fertilization occurs in the ampulla of the uterine tube • 200 million sperm cells enter the vaginal canal • Only about 10,000 make it to the uterine tubes • Less than 100 actually contact the egg • Only one will fertilize the egg © 2012 Pearson Education, Inc. Fertilization • Fertilization details • When the egg is ovulated, it is surrounded by the corona radiata, which protects the egg as it is being ovulated • Numerous sperm cells release hyaluronidase, from their acrosomal cap, in an effort -

Lymph Node Development Ontogeny of Stromal Organizer Cells During
Ontogeny of Stromal Organizer Cells during Lymph Node Development Cécile Bénézech, Andrea White, Emma Mader, Karine Serre, Sonia Parnell, Klaus Pfeffer, Carl F. Ware, Graham This information is current as Anderson and Jorge H. Caamaño of September 28, 2021. J Immunol 2010; 184:4521-4530; Prepublished online 17 March 2010; doi: 10.4049/jimmunol.0903113 http://www.jimmunol.org/content/184/8/4521 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2010/03/15/jimmunol.090311 Material 3.DC1 http://www.jimmunol.org/ References This article cites 47 articles, 22 of which you can access for free at: http://www.jimmunol.org/content/184/8/4521.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 28, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2010 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Ontogeny of Stromal Organizer Cells during Lymph Node Development Ce´cile Be´ne´zech,* Andrea White,* Emma Mader,* Karine Serre,* Sonia Parnell,* Klaus Pfeffer,† Carl F. -

Lymphangiogenesis and Angiogenesis During Human Fetal
Roost et al. Vascular Cell 2014, 6:22 http://www.vascularcell.com/content/6/1/22 VASCULAR CELL RESEARCH Open Access Lymphangiogenesis and angiogenesis during human fetal pancreas development Matthias S Roost1, Liesbeth van Iperen1, Ana de Melo Bernardo1, Christine L Mummery1, Françoise Carlotti2, Eelco JP de Koning2,3 and Susana M Chuva de Sousa Lopes1,4* Abstract Background: The complex endocrine and exocrine functionality of the human pancreas depends on an efficient fluid transport through the blood and the lymphatic vascular systems. The lymphatic vasculature has key roles in the physiology of the pancreas and in regulating the immune response, both important for developing successful transplantation and cell-replacement therapies to treat diabetes. However, little is known about how the lymphatic and blood systems develop in humans. Here, we investigated the establishment of these two vascular systems in human pancreas organogenesis in order to understand neovascularization in the context of emerging regenerative therapies. Methods: We examined angiogenesis and lymphangiogenesis during human pancreas development between 9 and 22 weeks of gestation (W9-W22) by immunohistochemistry. Results: As early as W9, the peri-pancreatic mesenchyme was populated by CD31-expressing blood vessels as well as LYVE1- and PDPN-expressing lymphatic vessels. The appearance of smooth muscle cell-coated blood vessels in the intra-pancreatic mesenchyme occurred only several weeks later and from W14.5 onwards the islets of Langerhans also became heavily irrigated by blood vessels. In contrast to blood vessels, LYVE1- and PDPN-expressing lymphatic vessels were restricted to the peri-pancreatic mesenchyme until later in development (W14.5-W17), and some of these invading lymphatic vessels contained smooth muscle cells at W17. -

The Rediscovery of the Lymphatic System: Old and New Insights Into the Development and Biological Function of the Lymphatic Vasculature
Downloaded from genesdev.cshlp.org on October 4, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature Guillermo Oliver1,3 and Michael Detmar2,3 1Department of Genetics, St. Jude Children’s Research Hospital, Memphis, Tennessee 38105, USA; 2Cutaneous Biology Research Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts 02114, USA The lymphatic system is composed of a vascular net- control lymphatic development and function. These work of thin-walled capillaries that drain protein-rich findings include the identification of specific genetic de- lymph from the extracellular spaces within most organs. fects in certain hereditary diseases that are associated A continuous single-cell layer of overlapping endothelial with lymphatic hypoplasia and dysfunction (i.e., lymph- cells lines the lymphatic capillaries, which lack a con- edemas; Milroy 1892; Meige 1898), and evidence that tinuous basement membrane and are, therefore, highly malignant tumors can directly activate lymphangiogen- permeable. Lymph returns to venous circulation via the esis and lymphatic metastasis (Karpanen et al. 2001; larger lymphatic collecting vessels, which contain a Mandriota et al. 2001; Skobe et al. 2001a; Stacker et al. muscular and adventitial layer, and the thoracic duct. 2001). The lymphatic system also includes lymphoid organs such as the lymph nodes, tonsils, Peyer’s patches, spleen, -

Secretion of Placental Growth Factor Promotes Angiogenesis by Enhanced Fibroblast-Like Synoviocytes Via Cadherin-11 Interaction
Interaction of Mesenchymal Stem Cells with Fibroblast-like Synoviocytes via Cadherin-11 Promotes Angiogenesis by Enhanced Secretion of Placental Growth Factor This information is current as of September 23, 2021. Su-Jung Park, Ki-Jo Kim, Wan-Uk Kim and Chul-Soo Cho J Immunol 2014; 192:3003-3010; Prepublished online 26 February 2014; doi: 10.4049/jimmunol.1302177 http://www.jimmunol.org/content/192/7/3003 Downloaded from References This article cites 50 articles, 15 of which you can access for free at: http://www.jimmunol.org/content/192/7/3003.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 23, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts Errata An erratum has been published regarding this article. Please see next page or: /content/192/10/4932.full.pdf The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2014 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Interaction of Mesenchymal Stem Cells with Fibroblast-like Synoviocytes via Cadherin-11 Promotes Angiogenesis by Enhanced Secretion of Placental Growth Factor Su-Jung Park,* Ki-Jo Kim,† Wan-Uk Kim,*,† and Chul-Soo Cho*,‡ Bone marrow–derived mesenchymal stem cells (MSC) exist in the synovium of patients with rheumatoid arthritis (RA), yet the role of MSC in RA is elusive.