The Lymphatic System in Human Embryos, with a Consideration of the Morphology of the System As a Whole
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

The Evolving Cardiac Lymphatic Vasculature in Development, Repair and Regeneration
REVIEWS The evolving cardiac lymphatic vasculature in development, repair and regeneration Konstantinos Klaourakis 1,2, Joaquim M. Vieira 1,2,3 ✉ and Paul R. Riley 1,2,3 ✉ Abstract | The lymphatic vasculature has an essential role in maintaining normal fluid balance in tissues and modulating the inflammatory response to injury or pathogens. Disruption of normal development or function of lymphatic vessels can have severe consequences. In the heart, reduced lymphatic function can lead to myocardial oedema and persistent inflammation. Macrophages, which are phagocytic cells of the innate immune system, contribute to cardiac development and to fibrotic repair and regeneration of cardiac tissue after myocardial infarction. In this Review, we discuss the cardiac lymphatic vasculature with a focus on developments over the past 5 years arising from the study of mammalian and zebrafish model organisms. In addition, we examine the interplay between the cardiac lymphatics and macrophages during fibrotic repair and regeneration after myocardial infarction. Finally, we discuss the therapeutic potential of targeting the cardiac lymphatic network to regulate immune cell content and alleviate inflammation in patients with ischaemic heart disease. The circulatory system of vertebrates is composed of two after MI. In this Review, we summarize the current complementary vasculatures, the blood and lymphatic knowledge on the development, structure and function vascular systems1. The blood vasculature is a closed sys- of the cardiac lymphatic vasculature, with an emphasis tem responsible for transporting gases, fluids, nutrients, on breakthroughs over the past 5 years in the study of metabolites and cells to the tissues2. This extravasation of cardiac lymphatic heterogeneity in mice and zebrafish. -

The Reproductive System: Embryology and Human Development
28 The Reproductive System: Embryology and Human Development PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • Development involves: • Differentiation of cells • Reorganization of cells • Development can be characterized by different periods of time • Prenatal development • Embryology • Postnatal development © 2012 Pearson Education, Inc. An Overview of Development • Development can be characterized by different periods of time • Prenatal development • Conception to delivery • Involves embryology (development during the prenatal period) • Postnatal development • Development from birth to maturity © 2012 Pearson Education, Inc. Fertilization • Fertilization is the joining of two haploid cells to create a diploid cell • Function of the haploid cells • Spermatozoon • Delivers the paternal chromosomes to the ovum • Ovum • Provides the maternal chromosomes • Provides nourishment for embryonic development © 2012 Pearson Education, Inc. Fertilization • Fertilization occurs in the ampulla of the uterine tube • 200 million sperm cells enter the vaginal canal • Only about 10,000 make it to the uterine tubes • Less than 100 actually contact the egg • Only one will fertilize the egg © 2012 Pearson Education, Inc. Fertilization • Fertilization details • When the egg is ovulated, it is surrounded by the corona radiata, which protects the egg as it is being ovulated • Numerous sperm cells release hyaluronidase, from their acrosomal cap, in an effort -

Lymph Node Development Ontogeny of Stromal Organizer Cells During
Ontogeny of Stromal Organizer Cells during Lymph Node Development Cécile Bénézech, Andrea White, Emma Mader, Karine Serre, Sonia Parnell, Klaus Pfeffer, Carl F. Ware, Graham This information is current as Anderson and Jorge H. Caamaño of September 28, 2021. J Immunol 2010; 184:4521-4530; Prepublished online 17 March 2010; doi: 10.4049/jimmunol.0903113 http://www.jimmunol.org/content/184/8/4521 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2010/03/15/jimmunol.090311 Material 3.DC1 http://www.jimmunol.org/ References This article cites 47 articles, 22 of which you can access for free at: http://www.jimmunol.org/content/184/8/4521.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 28, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2010 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Ontogeny of Stromal Organizer Cells during Lymph Node Development Ce´cile Be´ne´zech,* Andrea White,* Emma Mader,* Karine Serre,* Sonia Parnell,* Klaus Pfeffer,† Carl F. -

The Rediscovery of the Lymphatic System: Old and New Insights Into the Development and Biological Function of the Lymphatic Vasculature
Downloaded from genesdev.cshlp.org on October 4, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature Guillermo Oliver1,3 and Michael Detmar2,3 1Department of Genetics, St. Jude Children’s Research Hospital, Memphis, Tennessee 38105, USA; 2Cutaneous Biology Research Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts 02114, USA The lymphatic system is composed of a vascular net- control lymphatic development and function. These work of thin-walled capillaries that drain protein-rich findings include the identification of specific genetic de- lymph from the extracellular spaces within most organs. fects in certain hereditary diseases that are associated A continuous single-cell layer of overlapping endothelial with lymphatic hypoplasia and dysfunction (i.e., lymph- cells lines the lymphatic capillaries, which lack a con- edemas; Milroy 1892; Meige 1898), and evidence that tinuous basement membrane and are, therefore, highly malignant tumors can directly activate lymphangiogen- permeable. Lymph returns to venous circulation via the esis and lymphatic metastasis (Karpanen et al. 2001; larger lymphatic collecting vessels, which contain a Mandriota et al. 2001; Skobe et al. 2001a; Stacker et al. muscular and adventitial layer, and the thoracic duct. 2001). The lymphatic system also includes lymphoid organs such as the lymph nodes, tonsils, Peyer’s patches, spleen, -

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -

LAC.12 Embryology 2019-2020 Dr.Mahdi Alheety
LAC.12 Embryology 2019-2020 Dr.Mahdi ALheety Cardiovascular System Establishment of the Cardiogenic Field The vascular system appears in the middle of the third week, when the embryo is no longer able to satisfy its nutritional requirements by diffusion alone. Progenitor heart cells lie in the epiblast, immediately adjacent to the cranial end of the primitive streak. From there, they migrate through the streak and into the splanchnic layer of lateral plate mesoderm where they form a horseshoe-shaped cluster of cells called the primary heart field (PHF) cranial to the neural folds. As the progenitor heart cells migrate and form the PHF during days 16 to18, they are specified on both sides from lateral to medial to become the atria, left ventricle, and most of the right ventricle. Patterning of these cells occurs at the same time that laterality (left-right sidedness) is being established for the entire embryo and this process and the signaling pathway it is dependent upon is essential for normal heart development. The remainder of the heart, including part of the right ventricle and outflow tract (conus cordis and truncus arteriosus), is derived from the secondary heart field (SHF). This field of cells appears slightly later (days 20 to 21) than those in the PHF, resides in splanchnic mesoderm ventral to the posterior pharynx, and is responsible for lengthening the outflow tract. Cells in the SHF also exhibit laterality, such that those on the right side contribute to the left of the outflow tract region and those on the left contribute to the right. -
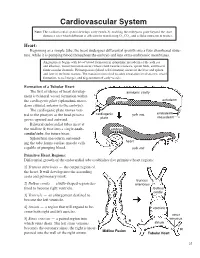
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle. -

Lymphangiogenesis Guidance by Paracrine and Pericellular Factors
Downloaded from genesdev.cshlp.org on October 10, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW Lymphangiogenesis guidance by paracrine and pericellular factors Kari Vaahtomeri,1 Sinem Karaman,1 Taija Mäkinen,2 and Kari Alitalo1 1Wihuri Research Institute, Translational Cancer Biology Program, Biomedicum Helsinki, University of Helsinki, FI-00014 Helsinki, Finland; 2Department of Immunology, Genetics, and Pathology, Uppsala University, 75185 Uppsala, Sweden Lymphatic vessels are important for tissue fluid homeo- in the downstream collector vessels (Bazigou and Maki- stasis, lipid absorption, and immune cell trafficking and nen 2013). are involved in the pathogenesis of several human diseas- With the exception of the Schlemm’s canal in the eyes, es. The mechanisms by which the lymphatic vasculature meningeal lymphatic vessels, and the majority of the (lac- network is formed, remodeled, and adapted to physiolog- teal) lymphatic vessels in the intestine, most lymphatic ical and pathological challenges are controlled by an intri- networks are generated during embryonic development cate balance of growth factor and biomechanical cues. (Kim et al. 2007; Aspelund et al. 2014, 2015; Kizhatil These transduce signals for the readjustment of gene ex- et al. 2014; Nurmi et al. 2015). However, they also under- pression and lymphatic endothelial migration, prolifera- go dynamic changes in adults. Lymphatic vessels can tion, and differentiation. In this review, we describe grow in length and caliber (lymphangiogenesis) in various several of these cues and how they are integrated for the pathological conditions, such as inflammation, wound generation of functional lymphatic vessel networks. healing, tumorigenesis, and in association with tissue transplantation. A common feature in many of these con- ditions is tissue edema and inflammation, which increase Some of the most dense lymphatic networks are located the demand for fluid drainage and immune cell traffick- under various epithelia that form the interface between ing. -

Lymphangiogenesis, Inflammation and Metastasis
ANTICANCER RESEARCH 25: 4503-4512 (2005) Review Lymphangiogenesis, Inflammation and Metastasis SEBASTIAN F. SCHOPPMANN Department of Surgery, Medical University of Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria Abstract. The lymphatic vascular system is necessary for the cells (1). In the periphery, antigen-presenting cells and return of extravasated interstitial fluid and macromolecules to lymphocytes enter the capillaries and migrate through the the blood circulation, for immune defense, and for the uptake lymphatic system to the lymph nodes to elicit acquired of dietary fats. Impaired functioning of lymphatic vessels results immune response in the body. In the small intestine, the in lymphedema, whereas tumor-associated lymphangiogenesis lymphatics play a special role in the process of fat may contribute to the spread of cancer cells from solid tumors. absorption. Recent studies have identified lymphatic molecular markers and This extensive drainage network is lined by a single, thin, growth factors necessary for lymphangiogenesis. In particular, non-fenestrated lymphatic endothelial cell (LECs) layer (2). lymphatic endothelial receptor tyrosine kinase VEGFR-3, and An incomplete basement membrane is characteristic, and the its ligands VEGF-C and VEGF-D, are major players in lymphatic endothelial cells are anchored to the extracellular promoting lymphatic vascular growth both during development matrix through elastic fibers, which keep the vessels open, and in pathological conditions. Lymphatic vessels play a crucial allowing for changes in interstitial pressure (3). role in a variety of human cancers, since invasion of lymphatic Two theories about the development of the lymphatic vessels by tumor cells and subsequent development of lymph system were proposed at the beginning of the last century: i) node metastases significantly influence the prognosis of cancer the venous origin of lymphatic vessels and ii) the de novo patients and, therefore, represent an integral part of tumor formation of primary lymph sacs in the mesenchyme (4, 5). -

2. Embryonic Development of the Lymphovascular System and Tumor Lymphangiogenesis
2. EMBRYONIC DEVELOPMENT OF THE LYMPHOVASCULAR SYSTEM AND TUMOR LYMPHANGIOGENESIS JÖRG WILTING, MARIA PAPOUTSI, KERSTIN BUTTLER, AND JÜRGEN BECKER Children’s Hospital, Pediatrics I, University of Goettingen, Robert-Koch-Strasse, Goettingen, Germany INTRODUCTION The embryonic development of the lymphatic vascular system starts considerably later than the blood vascular system. In chick embryos, the first blood vessels can be seen after 1 day of incubation, whereas morphological evidence for lymphatic endothelial cells (LECs) is present around day 5. However, with specific marker molecules, such as the transcription factor Prox1, LEC precursors can be identified in day-3.5 embryos. In the mouse, blood vessel development starts at embryonic day (ED) 7.5, whereas the anlagen of lymph vessels can be seen in the jugular region at ED 10. In human embryos there is a period of 3–4 weeks between the appearance of the first blood vas- cular endothelial cells (BECs) and LECs.There is good evidence that LECs develop from specialized parts of the venous system; however, there is growing evidence that scattered mesenchymal cells integrate into the growing fetal lymphatics. Similarly, lymphatics induced by tumors are derived mainly from local vessels, but, to some extent, pathologic lymphatics seem to develop by integration of circulating cells with lymphendothelial characteristics. In embryos, like in tumors, the most potent inducers of lymphangiogenesis are vascular endothelial growth factor (VEGF)-C and -D,which act mainly via VEGF receptor-3 (flt-4) on LECs.We have shown that blocking of this interaction prevents lymphangiogenesis in experimental A375 melanomas, while blocking of VEGF-A greatly inhibits blood vessel development (hemangiogenesis) in 18 Cancer Metastasis and the Lymphovascular System such tumors.