Dosage-Sensitive Requirement for Mouse Dll4 in Artery Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coronary Arterial Development Is Regulated by a Dll4-Jag1-Ephrinb2 Signaling Cascade
RESEARCH ARTICLE Coronary arterial development is regulated by a Dll4-Jag1-EphrinB2 signaling cascade Stanislao Igor Travisano1,2, Vera Lucia Oliveira1,2, Bele´ n Prados1,2, Joaquim Grego-Bessa1,2, Rebeca Pin˜ eiro-Sabarı´s1,2, Vanesa Bou1,2, Manuel J Go´ mez3, Fa´ tima Sa´ nchez-Cabo3, Donal MacGrogan1,2*, Jose´ Luis de la Pompa1,2* 1Intercellular Signalling in Cardiovascular Development and Disease Laboratory, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), Madrid, Spain; 2CIBER de Enfermedades Cardiovasculares, Madrid, Spain; 3Bioinformatics Unit, Centro Nacional de Investigaciones Cardiovasculares, Madrid, Spain Abstract Coronaries are essential for myocardial growth and heart function. Notch is crucial for mouse embryonic angiogenesis, but its role in coronary development remains uncertain. We show Jag1, Dll4 and activated Notch1 receptor expression in sinus venosus (SV) endocardium. Endocardial Jag1 removal blocks SV capillary sprouting, while Dll4 inactivation stimulates excessive capillary growth, suggesting that ligand antagonism regulates coronary primary plexus formation. Later endothelial ligand removal, or forced expression of Dll4 or the glycosyltransferase Mfng, blocks coronary plexus remodeling, arterial differentiation, and perivascular cell maturation. Endocardial deletion of Efnb2 phenocopies the coronary arterial defects of Notch mutants. Angiogenic rescue experiments in ventricular explants, or in primary human endothelial cells, indicate that EphrinB2 is a critical effector of antagonistic Dll4 and Jag1 functions in arterial morphogenesis. Thus, coronary arterial precursors are specified in the SV prior to primary coronary plexus formation and subsequent arterial differentiation depends on a Dll4-Jag1-EphrinB2 signaling *For correspondence: [email protected] (DMG); cascade. [email protected] (JLP) Competing interests: The authors declare that no Introduction competing interests exist. -

Abnormal Embryonic Lymphatic Vessel Development in Tie1 Hypomorphic Mice Xianghu Qu, Kevin Tompkins, Lorene E
© 2014. Published by The Company of Biologists Ltd | Development (2014) 141, 1417 doi:10.1242/dev.108969 CORRECTION Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice Xianghu Qu, Kevin Tompkins, Lorene E. Batts, Mira Puri and H. Scott Baldwin There was an error published in Development 137, 1285-1295. Author name H. Scott Baldwin was incomplete. The correct author list appears above. The authors apologise to readers for this mistake. 1417 RESEARCH ARTICLE 1285 Development 137, 1285-1295 (2010) doi:10.1242/dev.043380 © 2010. Published by The Company of Biologists Ltd Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice Xianghu Qu1, Kevin Tompkins1, Lorene E. Batts1, Mira Puri2 and Scott Baldwin1,3,* SUMMARY Tie1 is an endothelial receptor tyrosine kinase that is essential for development and maintenance of the vascular system; however, the role of Tie1 in development of the lymphatic vasculature is unknown. To address this question, we first documented that Tie1 is expressed at the earliest stages of lymphangiogenesis in Prox1-positive venous lymphatic endothelial cell (LEC) progenitors. LEC Tie1 expression is maintained throughout embryonic development and persists in postnatal mice. We then generated two lines of Tie1 mutant mice: a hypomorphic allele, which has reduced expression of Tie1, and a conditional allele. Reduction of Tie1 levels resulted in abnormal lymphatic patterning and in dilated and disorganized lymphatic vessels in all tissues examined and in impaired lymphatic drainage in embryonic skin. Homozygous hypomorphic mice also exhibited abnormally dilated jugular lymphatic vessels due to increased production of Prox1-positive LECs during initial lymphangiogenesis, indicating that Tie1 is required for the early stages of normal lymphangiogenesis. -

The Evolving Cardiac Lymphatic Vasculature in Development, Repair and Regeneration
REVIEWS The evolving cardiac lymphatic vasculature in development, repair and regeneration Konstantinos Klaourakis 1,2, Joaquim M. Vieira 1,2,3 ✉ and Paul R. Riley 1,2,3 ✉ Abstract | The lymphatic vasculature has an essential role in maintaining normal fluid balance in tissues and modulating the inflammatory response to injury or pathogens. Disruption of normal development or function of lymphatic vessels can have severe consequences. In the heart, reduced lymphatic function can lead to myocardial oedema and persistent inflammation. Macrophages, which are phagocytic cells of the innate immune system, contribute to cardiac development and to fibrotic repair and regeneration of cardiac tissue after myocardial infarction. In this Review, we discuss the cardiac lymphatic vasculature with a focus on developments over the past 5 years arising from the study of mammalian and zebrafish model organisms. In addition, we examine the interplay between the cardiac lymphatics and macrophages during fibrotic repair and regeneration after myocardial infarction. Finally, we discuss the therapeutic potential of targeting the cardiac lymphatic network to regulate immune cell content and alleviate inflammation in patients with ischaemic heart disease. The circulatory system of vertebrates is composed of two after MI. In this Review, we summarize the current complementary vasculatures, the blood and lymphatic knowledge on the development, structure and function vascular systems1. The blood vasculature is a closed sys- of the cardiac lymphatic vasculature, with an emphasis tem responsible for transporting gases, fluids, nutrients, on breakthroughs over the past 5 years in the study of metabolites and cells to the tissues2. This extravasation of cardiac lymphatic heterogeneity in mice and zebrafish. -

Glossary of Key Terms and Concepts - Chapter 8
Glossary of Key Terms and Concepts - Chapter 8 Angioblasts - These "vessel-forming cells" may arise from any kind of mesoderm except prechordal plate mesoderm. Angioblastic cords - Angiocysts coalesce to form short blind-ended angioblastic cords. Angioblastic plexuses - Angioblastic cords coalesce to form complex interconnected vascular networks or plexuses. Angiocysts - These vesicles are formed by angioblasts during the process of vasculogenesis. Angiogenesis - This is the mechanism whereby preexisting vessels lengthen or branch by sprouting. Aortic arches - These vessels have been modified in humans to form the great vessels of the thorax (also see Ch. 7). Axis arteries - These central arteries of the limbs are derived from the 7th intersegmental arteries (upper limb) and 5th lumbar intersegmental arteries (lower limb). Blood islands - Blood islands are cysts of angioblasts containing hemoblasts. These coalesce to form blood vessels in the yolk sac and also form the coronary vasculature. Branchial arches - These are the gill bars of fish. Homologous structures of humans are more appropriately named "pharyngeal" arches. Cardinal system of veins - These veins drain the head and neck and body wall and extremities of the embryo. Anterior cardinals drain the head and neck and the trunk and lower extremities are drained by paired posterior cardinals. The posterior cardinal veins are replaced by subcardinal and supracardinal veins during the second month. Coronary vessels - These vessels of the heart form from epicardium as subepicardial plexuses fuse with sprouts of the aorta and coronary sinus to form the coronary arteries and coronary veins respectively. Endothelial cells - These cells arise from angioblasts to form the initial vascular network. -

A Novel Therapeutic Antibody Targeting Dll4 Modulates Endothelial Cell Function and Angiogenesis in Vivo
Published OnlineFirst June 7, 2012; DOI: 10.1158/1535-7163.MCT-11-1027 Molecular Cancer Therapeutic Discovery Therapeutics MEDI0639: A Novel Therapeutic Antibody Targeting Dll4 Modulates Endothelial Cell Function and Angiogenesis In Vivo David W. Jenkins1, Sarah Ross2, Margaret Veldman-Jones2, Ian N. Foltz3, Brandon C. Clavette3, Kathy Manchulenko4, Cath Eberlein2, Jane Kendrew2, Philip Petteruti1, Song Cho4, Melissa Damschroder4, Li Peng4, Dawn Baker2, Neil R. Smith2, Hazel M. Weir2, David C. Blakey2, Vahe Bedian1, and Simon T. Barry2 Abstract The Notch signaling pathway has been implicated in cell fate determination and differentiation in many tissues. Accumulating evidence points toward a pivotal role in blood vessel formation, and the importance of the Delta-like ligand (Dll) 4-Notch1 ligand–receptor interaction has been shown in both physiological and tumor angiogenesis. Disruption of this interaction leads to a reduction in tumor growth as a result of an increase in nonfunctional vasculature leading to poor perfusion of the tumor. MEDI0639 is an investigational human therapeutic antibody that targets Dll4 to inhibit the interaction between Dll4 and Notch1. The antibody cross- reacts to cynomolgus monkey but not mouse species orthologues. In vitro MEDI0639 inhibits the binding of Notch1 to Dll4, interacting via a novel epitope that has not been previously described. Binding to this epitope translates into MEDI0639 reversing Notch1-mediated suppression of human umbilical vein endothelial cell growth in vitro. MEDI0639 administration resulted in stimulation of tubule formation in a three-dimensional (3D) endothelial cell outgrowth assay, a phenotype driven by disruption of the Dll4-Notch signaling axis. In contrast, in a two-dimensional endothelial cell–fibroblast coculture model, MEDI0639 is a potent inhibitor of tubule formation. -
Development of HEART 4-VEINS
Development of brachiocephalic veins 1. Right brachiocephalic vein is formed by cranial part of right anterior cardinal vein and 2. Left brachiocephalic is formed by cranial part of left anterior cardinal vein and the interant.cardinal anastomosis. Development of superior vena cava 1. The part up to the opening of vena azygos develops from caudal part of right ant.cardinal vein and 2. The part below the opening (intrapericardial part) is formed by the right common cardinal vein. Development of azygos and hemiazygos veins A. 1. Vena azygos develops from right azygos line vein and 2. The arch of vena azygos is formed by the cranial end of right postcardinal vein. B. Hemiazygos veins are formed by the left azygos line vein. Development of Inferior vena cava Inferior vena cava is formed, from below upwards by: 1. Begins by the union of the two common iliac veins (postcardinal veins), 2. Right supracardinal, 3. Right supra-subcardinal anastomosis, 4. Right subcardinal, 5. New formation (hepatic segment) and 6. Hepatocardiac channel (terminal part of right vitelline vein). Congenital anomalies • Double inferior vena cava • Absence • Left SVC • Double SVC DEVELOPMENT OF PORTAL VEIN 1. The portal vein is formed behind the neck of pancreas by the union of superior mesentric and splenic vein to the left vitelline vein. 2. The part of the portal vein which is behind the Ist part of duodenum is formed by middle dorsal transverse anastomosis. 3. Part of portal vein which is in the free margin of lesser omentum is formed by cranial or distal part of right vitelline vein. -

New Insights Into the Role of Notch Signaling in the Bone Marrow Ashley N
EDITORIALS Notch in the niche: new insights into the role of Notch signaling in the bone marrow Ashley N. Vanderbeck1-3 and Ivan Maillard2-4 1VMD-PhD program at University of Pennsylvania School of Veterinary Medicine; 2Immunology Graduate Group, University of Pennsylvania; 3Abramson Family Cancer Research Institute, University of Pennsylvania and 4Division of Hematology-Oncology, Department of Medicine, University of Pennsylvania, Philadelphia, PA, USA E-mail: IVAN MAILLARD - [email protected] doi:10.3324/haematol.2019.230854 n the bone marrow, specialized non-hematopoietic cells or radiation-induced myelosuppression relies heavily on form unique microenvironmental niches that support and regeneration of the endothelial cell network in order to sup- regulate the functions of hematopoietic stem and progen- port the hematopoietic compartment.6,15,16 By examining the I 1 itor cells (HSPC). Although many niche factors are well role of Notch signaling after injury using bone marrow defined, the role of Notch signaling remains controversial chimeras and genetic models of cell type-specific Notch inac- (see Figure 1). Notch signaling in HSPC has been reported to tivation, Shao et al. dissected the functional importance of regulate hematopoietic stem cell maintenance, suppress two possible routes of communication: cross-talk between myelopoiesis, and promote megakaryocyte/erythroid cell endothelial cells and HSPC (Figure 1, ቢ), as well as Notch development.2-7 Mechanistically, most previous reports have signaling between endothelial cells (Figure 1, ባ) that indi- been built on the concept that Notch receptors in HSPC rectly affects HSPC. First, the authors demonstrated that interact with Notch ligands expressed in niche endothelial endothelial restoration after bone marrow injury relied on cells, or alternatively in other components of the bone mar- activation of Notch signaling through the Notch1 receptor. -

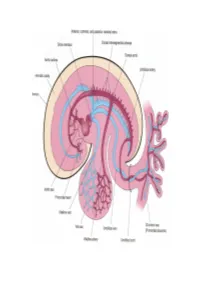
Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -

DLL4 Gene Delta Like Canonical Notch Ligand 4
DLL4 gene delta like canonical Notch ligand 4 Normal Function The DLL4 gene provides instructions for making a protein that is part of a signaling pathway known as the Notch pathway, which is important for normal development of many tissues throughout the body. The DLL4 protein attaches to a receptor protein called Notch1, fitting together like a key into its lock. When a connection is made between DLL4 and Notch1, a series of signaling reactions is launched (the Notch pathway), affecting cell functions. In particular, signaling stimulated by DLL4 plays a role in development of blood vessels before birth and growth of new blood vessels ( angiogenesis) throughout life. Health Conditions Related to Genetic Changes Adams-Oliver syndrome At least nine DLL4 gene mutations have been found in people with Adams-Oliver syndrome, a condition characterized by areas of missing skin (aplasia cutis congenita), usually on the scalp, and malformations of the hands and feet. Some of these mutations lead to production of an abnormally short protein that is likely broken down quickly, causing a shortage of DLL4. Other mutations change single protein building blocks ( amino acids) in the DLL4 protein. These changes are thought to alter the structure of the protein, impairing its ability to function. Loss of DLL4 function may underlie blood vessel abnormalities in people with Adams-Oliver syndrome; however, some people with DLL4-related Adams-Oliver syndrome do not have these abnormalities. It is not clear how loss of DLL4 function leads to the scalp and limb abnormalities characteristic of the condition. Researchers suggest these features may be due to abnormal blood vessel development before birth. -

Biological Roles of the Delta Family Notch Ligand Dll4 in Tumor and Endothelial Cells in Ovarian Cancer
Published OnlineFirst July 27, 2011; DOI: 10.1158/0008-5472.CAN-10-2719 Cancer Therapeutics, Targets, and Chemical Biology Research Biological Roles of the Delta Family Notch Ligand Dll4 in Tumor and Endothelial Cells in Ovarian Cancer Wei Hu1, Chunhua Lu1, Hee Dong Han1, Jie Huang1, De-yu Shen1, Rebecca L. Stone1, Alpa M. Nick1, Mian M.K. Shahzad1, Edna Mora1, Nicholas B. Jennings1, Sun Joo Lee1, Ju-Won Roh1, Koji Matsuo1, Masato Nishimura1, Blake W. Goodman1, Robert B. Jaffe6, Robert R. Langley2, Michael T. Deavers3, Gabriel Lopez-Berestein4, Robert L. Coleman1, and Anil K. Sood1,3,5 Abstract Emerging evidence suggests that the Notch/Delta-like ligand 4 (DLL4) pathway may offer important new targets for antiangiogenesis approaches. In this study, we investigated the clinical and biological significance of DLL4 in ovarian cancer. DLL4 was overexpressed in 72% of tumors examined in which it was an independent predictor of poor survival. Patients with tumors responding to anti-VEGF therapy had lower levels of DLL4 than patients with stable or progressive disease. Under hypoxic conditions, VEGF increased DLL4 expression in the tumor vasculature. Immobilized DLL4 also downregulated VEGFR2 expression in endothelial cells directly through methylation of the VEGFR2 promoter. RNAi-mediated silencing of DLL4 in ovarian tumor cells and tumor-associated endothelial cells inhibited cell growth and angiogenesis, accompanied by induction of hypoxia in the tumor microenvironment. Combining DLL4-targeted siRNA with bevacizumab resulted in greater inhibition of tumor growth, compared with control or treatment with bevacizumab alone. Together, our findings establish that DLL4 plays a functionally important role in both the tumor and endothelial compart- ments of ovarian cancer and that targeting DLL4 in combination with anti-VEGF treatment might improve outcomes of ovarian cancer treatment. -

Development of the Vascular System in Five to Twenty-One
THE DEVELOPMENT OF THE VASCULAR SYSTEM IN FIVE TO TWtNTY-ONE SOMITE DOG EMBRYOS by ELDEN WILLIAM MARTIN B, S., Kansas State College of Agriculture and ADolied Science, 195>U A THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Zoology KANSAS STATV: COLLEGE OF AGRICULTURE AND A PLIED SCIENCE 1958 LP TH Ooco/*>*Tv TABLE OF CONTENTS INTRO IXJ CTION AND LITERATURE REVIEW 1 MATERIALS AND METHODS ^ OBSERVATIONS 6 Five-Somi te Stag© . 6 Seven-Somite Stage 8 Eight-Somite Stage 9 Ten- and bleven-Somite Stage 12 Twe 1 ve-Somi te Stage • \\i Fifteen-Somite Stage 18 Seventeen-Somite Stage 21 Eighteen-Somite Stage 2$ Twenty- and Twenty- one -Somite Stage 27 INTERPRETATIONS AND DISCUSSION 30 Vasculogenesis • 30 Cardiogenesis 33 The Origin and Development of Arteries \ 3lj. Aortic Arches •••« 3I4. Cranial Arterie s ...•• 36 The Dorsal Aorta 37 Intersegmental AAteries 39 Vertebral Arteries 39 Vitelline Arteries }±q The Allantoic Artery \±\ Ill IITERPRETATION AND DISCUSSION (Contd.) The Origin and Development of Veins •• kl The Anterior Cardinal Veins . I4.I Posterior Cardinal Veins k2 Umbilical Veins U3 Common Cardinal Veins kh Interconnecting Vessels Ui> SUMMARY kl LITERA°URE CITED $1 ACKNOWLEDGMENTS 53 APPENDIX 5U HTmDUCTIOW AND LITFRATORF. rfvibw While the dog has been employed extensively as a labora- tory animal in various fields of scientific endeavour, the use of this animal in embryology has been neglected. As a con- sequence, the literature on the circulatory system of the dog was represented only by an unpublished thesis by Duffey (3) on oardlogenesis and the first heart movements. -

Development-Of-Heart
ANATOMY EMBRYOLOGY EMBRYOLOGY DEVLOPMENT OF THE HEART Normal Heart Structure Heart Anatomy HEART Normal Anatomy Contents Development of the Heart Tube Derivatives of the Heart Tube Cardiac Looping Development Heart Partition of The Heart Formation of Cardiac Valves Formation of AV Septum Foetal Circulation Formation of Aortic Arch System The cardiovascular system is the first system to function in the Embryo. Starts developing in the middle of the third week, and heart becomes functional at the beginning of fourth week Development The cells of the splanchnic of Heart mesoderm in the cardiogenic area proliferate to form the angiogenetic clusters. The canalization of angiogenetic clusters in the splanchnic mesoderm of cardiogenic area results in the formation of the paired heart tubes. Day 16 heart heart field (PHF). progenitor PHF gives rise to cells atria, migrate to area cranial to left ventricle and neural part of right folds, horseshoe- ventricle shaped region of Secondary heart Development splanchnic field (SHF) forms lateral of the Heart plate the mesoderm. This is remainder of R. primary Ventricle, Conus Cordis Truncus Arteriosus Development of the Heart Diagrammatic Development of the Heart Development of the Heart Development of the Heart Tube 1. Foregut 2. Intraembryonic coelom 3. Heart tubes 4. Dorsal mesocardium 5. Epimyocardium 6. Neural groove 7. Neural crest 8. Notochord 9. Dorsal aorta SHF lies posterior to Pharynx in splanchnic mesoderm, is regulated by neural crest cells that migrate through pharyngeal arches in this region