A Crucial Role of Caldesmon in Vascular Development in Vivo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coronary Arterial Development Is Regulated by a Dll4-Jag1-Ephrinb2 Signaling Cascade
RESEARCH ARTICLE Coronary arterial development is regulated by a Dll4-Jag1-EphrinB2 signaling cascade Stanislao Igor Travisano1,2, Vera Lucia Oliveira1,2, Bele´ n Prados1,2, Joaquim Grego-Bessa1,2, Rebeca Pin˜ eiro-Sabarı´s1,2, Vanesa Bou1,2, Manuel J Go´ mez3, Fa´ tima Sa´ nchez-Cabo3, Donal MacGrogan1,2*, Jose´ Luis de la Pompa1,2* 1Intercellular Signalling in Cardiovascular Development and Disease Laboratory, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), Madrid, Spain; 2CIBER de Enfermedades Cardiovasculares, Madrid, Spain; 3Bioinformatics Unit, Centro Nacional de Investigaciones Cardiovasculares, Madrid, Spain Abstract Coronaries are essential for myocardial growth and heart function. Notch is crucial for mouse embryonic angiogenesis, but its role in coronary development remains uncertain. We show Jag1, Dll4 and activated Notch1 receptor expression in sinus venosus (SV) endocardium. Endocardial Jag1 removal blocks SV capillary sprouting, while Dll4 inactivation stimulates excessive capillary growth, suggesting that ligand antagonism regulates coronary primary plexus formation. Later endothelial ligand removal, or forced expression of Dll4 or the glycosyltransferase Mfng, blocks coronary plexus remodeling, arterial differentiation, and perivascular cell maturation. Endocardial deletion of Efnb2 phenocopies the coronary arterial defects of Notch mutants. Angiogenic rescue experiments in ventricular explants, or in primary human endothelial cells, indicate that EphrinB2 is a critical effector of antagonistic Dll4 and Jag1 functions in arterial morphogenesis. Thus, coronary arterial precursors are specified in the SV prior to primary coronary plexus formation and subsequent arterial differentiation depends on a Dll4-Jag1-EphrinB2 signaling *For correspondence: [email protected] (DMG); cascade. [email protected] (JLP) Competing interests: The authors declare that no Introduction competing interests exist. -

Abnormal Embryonic Lymphatic Vessel Development in Tie1 Hypomorphic Mice Xianghu Qu, Kevin Tompkins, Lorene E
© 2014. Published by The Company of Biologists Ltd | Development (2014) 141, 1417 doi:10.1242/dev.108969 CORRECTION Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice Xianghu Qu, Kevin Tompkins, Lorene E. Batts, Mira Puri and H. Scott Baldwin There was an error published in Development 137, 1285-1295. Author name H. Scott Baldwin was incomplete. The correct author list appears above. The authors apologise to readers for this mistake. 1417 RESEARCH ARTICLE 1285 Development 137, 1285-1295 (2010) doi:10.1242/dev.043380 © 2010. Published by The Company of Biologists Ltd Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice Xianghu Qu1, Kevin Tompkins1, Lorene E. Batts1, Mira Puri2 and Scott Baldwin1,3,* SUMMARY Tie1 is an endothelial receptor tyrosine kinase that is essential for development and maintenance of the vascular system; however, the role of Tie1 in development of the lymphatic vasculature is unknown. To address this question, we first documented that Tie1 is expressed at the earliest stages of lymphangiogenesis in Prox1-positive venous lymphatic endothelial cell (LEC) progenitors. LEC Tie1 expression is maintained throughout embryonic development and persists in postnatal mice. We then generated two lines of Tie1 mutant mice: a hypomorphic allele, which has reduced expression of Tie1, and a conditional allele. Reduction of Tie1 levels resulted in abnormal lymphatic patterning and in dilated and disorganized lymphatic vessels in all tissues examined and in impaired lymphatic drainage in embryonic skin. Homozygous hypomorphic mice also exhibited abnormally dilated jugular lymphatic vessels due to increased production of Prox1-positive LECs during initial lymphangiogenesis, indicating that Tie1 is required for the early stages of normal lymphangiogenesis. -

CCM2 and CCM3 Proteins Contribute to Vasculogenesis and Angiogenesis in Human Placenta
Histol Histopathol (2009) 24: 1287-1294 Histology and http://www.hh.um.es Histopathology Cellular and Molecular Biology CCM2 and CCM3 proteins contribute to vasculogenesis and angiogenesis in human placenta Gamze Tanriover1, Yasemin Seval1, Leyla Sati1, Murat Gunel2 and Necdet Demir1 1Department of Histology and Embryology, Akdeniz University, School of Medicine, Antalya, Turkey and 2 Department of Neurosurgery, Yale University, School of Medicine, New Haven, CT, USA Summary. Placenta as an ideal model to study Introduction angiogenic mechanisms have been established in previous studies. There are two processes, The placenta is a multifaceted organ that plays a vasculogenesis and angiogenesis, involved in blood critical role in maintaining and protecting the developing vessel formation during placental development. fetus. Normal development and function of the placenta Therefore, blood vessel formation is a crucial issue that requires extensive vasculogenesis and subsequent might cause vascular malformations. One of the vascular angiogenesis, in both maternal and fetal tissues. malformations is cerebral cavernous malformation Vasculogenesis is the formation of the primitive vascular (CCM) in the central nervous system, consisting of network de novo from progenitor cells, and angiogenesis endothelium-lined vascular channels without intervening is identified as the extension of blood vessels from normal brain parenchyma. Three CCM loci have been preexisting vascular structures (Demir et al., 1989, 2006; mapped as Ccm1, Ccm2, Ccm3 genes in CCM. In order Geva et al., 2002; Charnock-Jones et al., 2004). Many to investigate whether CCM proteins participate in blood factors, such as vascular endothelial growth factor vessel formation, we report here the expression patterns (VEGF), angiopoietins (Angpt-1 and -2) and their of CCM2 and CCM3 in developing and term human receptors are involved in the molecular regulation of placenta by means of immunohistochemistry and these diverse developmental steps. -

Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease
Journal of Cardiovascular Development and Disease Review Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease Laura A. Dyer 1 and Sandra Rugonyi 2,* 1 Department of Biology, University of Portland, Portland, OR 97203, USA; [email protected] 2 Department of Biomedical Engineering, Oregon Health & Science University, Portland, OR 97239, USA * Correspondence: [email protected] Abstract: In congenital heart disease, the presence of structural defects affects blood flow in the heart and circulation. However, because the fetal circulation bypasses the lungs, fetuses with cyanotic heart defects can survive in utero but need prompt intervention to survive after birth. Tetralogy of Fallot and persistent truncus arteriosus are two of the most significant conotruncal heart defects. In both defects, blood access to the lungs is restricted or non-existent, and babies with these critical conditions need intervention right after birth. While there are known genetic mutations that lead to these critical heart defects, early perturbations in blood flow can independently lead to critical heart defects. In this paper, we start by comparing the fetal circulation with the neonatal and adult circulation, and reviewing how altered fetal blood flow can be used as a diagnostic tool to plan interventions. We then look at known factors that lead to tetralogy of Fallot and persistent truncus arteriosus: namely early perturbations in blood flow and mutations within VEGF-related pathways. The interplay between physical and genetic factors means that any one alteration can cause significant disruptions during development and underscore our need to better understand the effects of both blood flow and flow-responsive genes. -

Extensive Vasculogenesis, Angiogenesis, and Organogenesis Precede Lethality in Mice Lacking All V Integrins
Cell, Vol. 95, 507–519, November 13, 1998, Copyright ©1998 by Cell Press Extensive Vasculogenesis, Angiogenesis, and Organogenesis Precede Lethality in Mice Lacking All ␣v Integrins Bernhard L. Bader,*‡ Helen Rayburn,* their ligands. Significant expression of ␣v integrins has Denise Crowley,* and Richard O. Hynes*† been noted, in particular, in neural crest cells (Delannet * Howard Hughes Medical Institute et al., 1994), glial cells (Hirsch et al., 1994; Milner and Center for Cancer Research ffrench-Constant, 1994), muscle (Hirsch et al., 1994; Mc- and Department of Biology Donald et al., 1995; Martin and Sanes, 1997), osteoclasts Massachusetts Institute of Technology (Va¨ a¨ na¨ nen and Horton, 1995), epithelia (␣v6; Breuss et Cambridge, Massachusetts 02139 al., 1995; Huang et al., 1996), and blood vessels during development (Brooks et al., 1994a; Drake et al., 1995; Friedlander et al., 1995, 1996) or angiogenesis in re- Summary sponse to tumors (Brooks et al., 1994a, 1994b, 1996, 1998; Varner et al., 1995). ␣v integrins have been implicated in many develop- Among the ligands for various ␣v integrins is fibro- mental processes and are therapeutic targets for inhi- nectin (FN), and results on mouse embryos lacking FN bition of angiogenesis and osteoporosis. Surprisingly, or FN receptor integrins suggest that ␣v integrins might ablation of the gene for the ␣v integrin subunit, elimi- be important receptors for FN during early development. nating all five ␣v integrins, although causing lethality, FN-null embryos fail to form notochord or somites allows considerable development and organogenesis (George et al., 1993; Georges-Labouesse et al., 1996), including, most notably, extensive vasculogenesis and whereas embryos null for either (Yang et al., 1993, 1995) angiogenesis. -

Copyright by Steven A. Vokes 2002 the Dissertation Committee for Steven Alexander Vokes Certifies That This Is the Approved Version of the Following Dissertation
Copyright by Steven A. Vokes 2002 The Dissertation Committee for Steven Alexander Vokes Certifies that this is the approved version of the following dissertation: The Role of Endoderm in Vascular Patterning Committee: Janice A. Fischer, Supervisor Paul A. Krieg , Co-Supervisor Alan M. Lloyd Arlen W. Johnson S. Martin Shankland R. Adron Harris The Role of Endoderm in Vascular Patterning by Steven Alexander Vokes, B.A. Dissertation Presented to the Faculty of the Graduate School of The University of Texas at Austin in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy The University of Texas at Austin December, 2002 Dedication This work, symbolic of my higher education, is dedicated to my parents Carol and Emmett Vokes, who played such an integral role in its foundations. Acknowledgements I have been extremely fortunate to have excellent mentoring during my time in graduate school. I thank Paul Krieg for his suggestions, enthusiasm, encouragement and friendship during this learning process. He has taught me how to think (and write) critically and insightfully about science. I am also grateful to Amy Cheng Vollmer, my undergraduate mentor. She introduced me to the joys of scientific research, and continues to give me excellent advice whenever I need it most. I thank Peter Vize, whose conversations led to the first experiments described within. In daily interactions, I have benefited from a caste of talented co-workers and advisors. These include Craig Newman, Wendy Gerber, Ondine Cleaver, Tom Carroll, Eric Small, Rob Garriock, Martha Joe, Parker Antin, Ray Runyan, Jean Wilson, Carol Gregorio and all past and present members of the Krieg lab. -

The Evolving Cardiac Lymphatic Vasculature in Development, Repair and Regeneration
REVIEWS The evolving cardiac lymphatic vasculature in development, repair and regeneration Konstantinos Klaourakis 1,2, Joaquim M. Vieira 1,2,3 ✉ and Paul R. Riley 1,2,3 ✉ Abstract | The lymphatic vasculature has an essential role in maintaining normal fluid balance in tissues and modulating the inflammatory response to injury or pathogens. Disruption of normal development or function of lymphatic vessels can have severe consequences. In the heart, reduced lymphatic function can lead to myocardial oedema and persistent inflammation. Macrophages, which are phagocytic cells of the innate immune system, contribute to cardiac development and to fibrotic repair and regeneration of cardiac tissue after myocardial infarction. In this Review, we discuss the cardiac lymphatic vasculature with a focus on developments over the past 5 years arising from the study of mammalian and zebrafish model organisms. In addition, we examine the interplay between the cardiac lymphatics and macrophages during fibrotic repair and regeneration after myocardial infarction. Finally, we discuss the therapeutic potential of targeting the cardiac lymphatic network to regulate immune cell content and alleviate inflammation in patients with ischaemic heart disease. The circulatory system of vertebrates is composed of two after MI. In this Review, we summarize the current complementary vasculatures, the blood and lymphatic knowledge on the development, structure and function vascular systems1. The blood vasculature is a closed sys- of the cardiac lymphatic vasculature, with an emphasis tem responsible for transporting gases, fluids, nutrients, on breakthroughs over the past 5 years in the study of metabolites and cells to the tissues2. This extravasation of cardiac lymphatic heterogeneity in mice and zebrafish. -

Endothelium in the Pharyngeal Arches 3, 4 and 6 Is Derived from the Second Heart Field
Thomas Jefferson University Jefferson Digital Commons Center for Translational Medicine Faculty Papers Center for Translational Medicine 1-15-2017 Endothelium in the pharyngeal arches 3, 4 and 6 is derived from the second heart field. Xia Wang Thomas Jefferson University Dongying Chen Thomas Jefferson University Kelley Chen Thomas Jefferson University Ali Jubran Thomas Jefferson University AnnJosette Ramirez Thomas Jefferson University Follow this and additional works at: https://jdc.jefferson.edu/transmedfp Part of the Translational Medical Research Commons LetSee next us page know for additional how authors access to this document benefits ouy Recommended Citation Wang, Xia; Chen, Dongying; Chen, Kelley; Jubran, Ali; Ramirez, AnnJosette; and Astrof, Sophie, "Endothelium in the pharyngeal arches 3, 4 and 6 is derived from the second heart field." (2017). Center for Translational Medicine Faculty Papers. Paper 45. https://jdc.jefferson.edu/transmedfp/45 This Article is brought to you for free and open access by the Jefferson Digital Commons. The Jefferson Digital Commons is a service of Thomas Jefferson University's Center for Teaching and Learning (CTL). The Commons is a showcase for Jefferson books and journals, peer-reviewed scholarly publications, unique historical collections from the University archives, and teaching tools. The Jefferson Digital Commons allows researchers and interested readers anywhere in the world to learn about and keep up to date with Jefferson scholarship. This article has been accepted for inclusion in Center for Translational Medicine Faculty Papers by an authorized administrator of the Jefferson Digital Commons. For more information, please contact: [email protected]. Authors Xia Wang, Dongying Chen, Kelley Chen, Ali Jubran, AnnJosette Ramirez, and Sophie Astrof This article is available at Jefferson Digital Commons: https://jdc.jefferson.edu/transmedfp/45 HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Dev Biol Manuscript Author . -

Glossary of Key Terms and Concepts - Chapter 8
Glossary of Key Terms and Concepts - Chapter 8 Angioblasts - These "vessel-forming cells" may arise from any kind of mesoderm except prechordal plate mesoderm. Angioblastic cords - Angiocysts coalesce to form short blind-ended angioblastic cords. Angioblastic plexuses - Angioblastic cords coalesce to form complex interconnected vascular networks or plexuses. Angiocysts - These vesicles are formed by angioblasts during the process of vasculogenesis. Angiogenesis - This is the mechanism whereby preexisting vessels lengthen or branch by sprouting. Aortic arches - These vessels have been modified in humans to form the great vessels of the thorax (also see Ch. 7). Axis arteries - These central arteries of the limbs are derived from the 7th intersegmental arteries (upper limb) and 5th lumbar intersegmental arteries (lower limb). Blood islands - Blood islands are cysts of angioblasts containing hemoblasts. These coalesce to form blood vessels in the yolk sac and also form the coronary vasculature. Branchial arches - These are the gill bars of fish. Homologous structures of humans are more appropriately named "pharyngeal" arches. Cardinal system of veins - These veins drain the head and neck and body wall and extremities of the embryo. Anterior cardinals drain the head and neck and the trunk and lower extremities are drained by paired posterior cardinals. The posterior cardinal veins are replaced by subcardinal and supracardinal veins during the second month. Coronary vessels - These vessels of the heart form from epicardium as subepicardial plexuses fuse with sprouts of the aorta and coronary sinus to form the coronary arteries and coronary veins respectively. Endothelial cells - These cells arise from angioblasts to form the initial vascular network. -
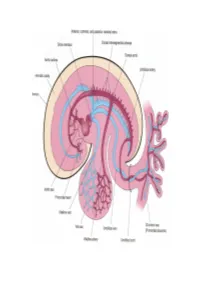
Development of HEART 4-VEINS
Development of brachiocephalic veins 1. Right brachiocephalic vein is formed by cranial part of right anterior cardinal vein and 2. Left brachiocephalic is formed by cranial part of left anterior cardinal vein and the interant.cardinal anastomosis. Development of superior vena cava 1. The part up to the opening of vena azygos develops from caudal part of right ant.cardinal vein and 2. The part below the opening (intrapericardial part) is formed by the right common cardinal vein. Development of azygos and hemiazygos veins A. 1. Vena azygos develops from right azygos line vein and 2. The arch of vena azygos is formed by the cranial end of right postcardinal vein. B. Hemiazygos veins are formed by the left azygos line vein. Development of Inferior vena cava Inferior vena cava is formed, from below upwards by: 1. Begins by the union of the two common iliac veins (postcardinal veins), 2. Right supracardinal, 3. Right supra-subcardinal anastomosis, 4. Right subcardinal, 5. New formation (hepatic segment) and 6. Hepatocardiac channel (terminal part of right vitelline vein). Congenital anomalies • Double inferior vena cava • Absence • Left SVC • Double SVC DEVELOPMENT OF PORTAL VEIN 1. The portal vein is formed behind the neck of pancreas by the union of superior mesentric and splenic vein to the left vitelline vein. 2. The part of the portal vein which is behind the Ist part of duodenum is formed by middle dorsal transverse anastomosis. 3. Part of portal vein which is in the free margin of lesser omentum is formed by cranial or distal part of right vitelline vein. -

Azygos Vein System Abnormality: Case Report
Gülhane Týp Dergisi 2006; 48: 180-182 OLGU SUNUMU © Gülhane Askeri Týp Akademisi 2006 Azygos vein system abnormality: case report Necdet Kocabýyýk (*), Tunç Kutoðlu (**), Soner Albay (*), Bülent Yalçýn (*), Hasan Ozan (*) Summary Introduction Variations seen in the thoracic vein system are Abnormalities related to the azygos system are not rare (1). In a series related to the development of these veins. of 200 cases, Bergman et al. have reported the incidence of this anomaly During the dissection from the posterior medi- astinum of the 60-year-old male cadaver, it 26% (2). These abnormalities are generally explained by the embryolog- was observed that there was no complete ical development. Venous branching of the azygos vein varies (3). There accessory hemiazygos vein, and both posterior are two origins of the azygos and hemiazygos veins. By union of these intercostal veins and hemiazygos vein (above origins and regression of some parts, azygos system comes into its final T10 level) drained bilaterally to the azygos vein. Considering these types of variations is status (4). Different types of structures may occur when these veins important during imaging this region and surgi- develop. Abnormalities about azygos system and especially the variations cal operations. of the hemiazygos veins are not clearly described in the literature. In this Key words: Azygos vein, hemiazygos vein, superior vena cava, venous anomaly presentation absence of the accessory hemiazygos vein and possible causes of these types of variations are discussed in view of the embry- Özet ological development. Azigos ven sistem anomalisi: olgu sunumu Toraks ven sisteminde görülen varyasyonlar, embriyolojik olarak bu venlerin geliþimiyle ilgi- Case Report lidir. -

Secretion of Placental Growth Factor Promotes Angiogenesis by Enhanced Fibroblast-Like Synoviocytes Via Cadherin-11 Interaction
Interaction of Mesenchymal Stem Cells with Fibroblast-like Synoviocytes via Cadherin-11 Promotes Angiogenesis by Enhanced Secretion of Placental Growth Factor This information is current as of September 23, 2021. Su-Jung Park, Ki-Jo Kim, Wan-Uk Kim and Chul-Soo Cho J Immunol 2014; 192:3003-3010; Prepublished online 26 February 2014; doi: 10.4049/jimmunol.1302177 http://www.jimmunol.org/content/192/7/3003 Downloaded from References This article cites 50 articles, 15 of which you can access for free at: http://www.jimmunol.org/content/192/7/3003.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 23, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts Errata An erratum has been published regarding this article. Please see next page or: /content/192/10/4932.full.pdf The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2014 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Interaction of Mesenchymal Stem Cells with Fibroblast-like Synoviocytes via Cadherin-11 Promotes Angiogenesis by Enhanced Secretion of Placental Growth Factor Su-Jung Park,* Ki-Jo Kim,† Wan-Uk Kim,*,† and Chul-Soo Cho*,‡ Bone marrow–derived mesenchymal stem cells (MSC) exist in the synovium of patients with rheumatoid arthritis (RA), yet the role of MSC in RA is elusive.