Description of Merluccius Tasmanicus Sp. Nov. and Redescription
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MERLUZA COMÚN Merluccius Gayi Gayi (Guichenot, 1848)
Ficha Pesquera Noviembre - 2008 MERLUZA COMÚN Merluccius gayi gayi (Guichenot, 1848) I. ANTECEDENTES DEL RECURSO Antecedentes biológicos Familia Merlucciidae Orden Gadiformes Clase Actinopterygii Hábitat Batidemersal Alimentación Zooplancton (eufausidos), Necton (peces juveniles), Zoobentos (crustáceos decápodos). Canibalismo Tamaño máximo (cm) 80 cm LT Talla modal 2008 (cm) 35 cm LT (industrial); 35 cm LT (espinel); 37 cm LT (enmalle) Longevidad (años) 15 años Edad de reclutamiento 2-3 años Ciclo de vida El ciclo de vida de esta especie está fuertemente asociado a la columna de agua sobre el área de la plataforma y talud continental de Chile centro-sur (zona nerítica), aunque bajo circunstancias ambientales extraordinarias es posible que ciertos procesos se verifiquen en la zona oceánica aledaña. El ciclo de vida comienza con el desove, el cual se realiza durante todo el año (desovante parcial) aunque el período de mayor intensidad se verifica en invierno-primavera, y un período de desove secundario en febrero- marzo de cada año. Las principales áreas de desove están cercanas a la costa entre Papudo (32º30’ LS) y Bahía San Pedro (40º50’ LS), los huevos desovados son fecundados en el área demersal de la zona nerítica, pasando las larvas a formar parte del necton por un periodo hasta el momento no determinado, y estando sujetas a los típicos procesos de transporte y advección que ocurren a lo largo de la zona centro sur de Chile. Después de un año a un año y medio, los juveniles de merluza común se reclutan al stock, habitando en áreas cercanas a la costa. A partir de los 3 años (34 cm LT), los ejemplares se reclutan a la pesquería y a partir de los 3,5 años (35-37 cm LT) alcanzan la edad de madurez sexual al 50%, constituyéndose en parte del stock adulto, el cual esta asociado a la contracorriente subsuperficial de Chile y Perú (Corriente de Gunther). -

ILLEGAL FISHING Which Fish Species Are at Highest Risk from Illegal and Unreported Fishing?
ILLEGAL FISHING Which fish species are at highest risk from illegal and unreported fishing? October 2015 CONTENTS EXECUTIVE SUMMARY 3 INTRODUCTION 4 METHODOLOGY 5 OVERALL FINDINGS 9 NOTES ON ESTIMATES OF IUU FISHING 13 Tunas 13 Sharks 14 The Mediterranean 14 US Imports 15 CONCLUSION 16 CITATIONS 17 OCEAN BASIN PROFILES APPENDIX 1: IUU Estimates for Species Groups and Ocean Regions APPENDIX 2: Estimates of IUU Risk for FAO Assessed Stocks APPENDIX 3: FAO Ocean Area Boundary Descriptions APPENDIX 4: 2014 U.S. Edible Imports of Wild-Caught Products APPENDIX 5: Overexploited Stocks Categorized as High Risk – U.S. Imported Products Possibly Derived from Stocks EXECUTIVE SUMMARY New analysis by World Wildlife Fund (WWF) finds that over 85 percent of global fish stocks can be considered at significant risk of Illegal, Unreported, and Unregulated (IUU) fishing. This evaluation is based on the most recent comprehensive estimates of IUU fishing and includes the worlds’ major commercial stocks or species groups, such as all those that are regularly assessed by the United Nations Food and Agriculture Organization (FAO). Based on WWF’s findings, the majority of the stocks, 54 percent, are categorized as at high risk of IUU, with an additional 32 perent judged to be at moderate risk. Of the 567 stocks that were assessed, the findings show that 485 stocks fall into these two categories. More than half of the world’s most overexploited stocks are at the highest risk of IUU fishing. Examining IUU risk by location, the WWF analysis shows that in more than one-third of the world’s ocean basins as designated by the FAO, all of these stocks were at high or moderate risk of IUU fishing. -

Otolith Trace Elemental Analyses of South American Austral Hake, Merluccius Australis
RESEARCH ARTICLE Otolith Trace Elemental Analyses of South American Austral Hake, Merluccius australis (Hutton, 1872) Indicates Complex Salinity Structuring on their Spawning/Larval Grounds Paul Brickle1,2,3*, Pia C. Schuchert4, Alexander I. Arkhipkin1, Malcolm R. Reid5, Haseeb S. Randhawa6 1 Directorate of Natural Resources, Fisheries Department, Falkland Islands Government, Stanley, Falkland Islands, 2 South Atlantic Environmental Research Institute, Stanley Cottage, Stanley, Falkland Islands, 3 School of Biological Sciences, University of Aberdeen, Zoology Building, Tillydrone Avenue, Aberdeen, AB24 2TZ, United Kingdom, 4 School of Biology, Newcastle University, Newcastle upon Tyne, NE1 7RU, United Kingdom, 5 Department of Chemistry, University of Otago, PO Box 56, Dunedin, 9054, New Zealand, 6 Ecology Degree Programme, Department of Botany, University of Otago, PO Box 56, Dunedin, 9054, New Zealand * [email protected] OPEN ACCESS Citation: Brickle P, Schuchert PC, Arkhipkin AI, Reid Abstract MR, Randhawa HS (2016) Otolith Trace Elemental Analyses of South American Austral Hake, Trace element signatures of otolith edges and cores from 335 austral hake (Merluccius Merluccius australis (Hutton, 1872) Indicates autralis) were analysed using LA-ICPMS from samples collected in Chilean and Falkland Complex Salinity Structuring on their Spawning/ Larval Grounds. PLoS ONE 11(1): e0145479. Islands' waters, in order to provide potential insights into stock discrimination and migra- doi:10.1371/journal.pone.0145479 tions. Fish were caught in two locations in Chile and four locations in the south-west of the Editor: Heather M. Patterson, Department of Falkland Islands Shelf. Univariate and multivariate analyses of trace element signatures in Agriculture and Water Resources, AUSTRALIA the edges of otoliths, representing adult fish, were not able to distinguish between samples ’ Received: July 27, 2014 collected in Chile and the Falkland Islands. -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -

4. Bibliografía
44 FAO Catálogo de Especies para los Fines de la Pesca No 2 4. BIBLIOGRAFÍA Abe, T. y Funabashi, M. 1993. A record of a Merlucciid Fish from off Ibaraki Prefecture, Japón. UO, 42: 1-8. Adams, A. 1864. Manual Nat. Hist.: 194. En: G.B. Goode y T.H. Bean, (1895): 386. Agassiz, A. 1988. Three cruises of the United States coast and feodetic survey steamer “Blake”. [Chapter xv. Sketches of the characteristic deep-sea types. Fishes: 21-36. Figs. 195-224 is by Goode y Bean.] Bull. Mus. Comp. Zool. Harvard, 15(en 2 vols.): i-xxii + 1-314 y 1- 220. Aguayo, M. 1995. Biology and fisheries of Chilean hakes (M. gayi and M. australis). En J. Alheit y T.J. Pitcher, eds. Hake: Fisheries, ecology and markets. Londres, Chapman y Hall. Fish and Fisheries Series, p. 305-337. Ahlstrom, E.H. y Counts, R.C. 1955. Eggs and larvae of the Pacific hake Merluccius productus. U.S. Fish Wildl. Serv. Fish. Bull., 99(56): 294-329. Alarcón, R. y Arancibia, H. 1993. Talla de primera madurez sexual y fecundidad parcial en la merluza común, Merluccius gayi gayi (Guichenot, 1848). Cienc. Tec. Mar, Cona, 16: 33-45. Alheit, J. y Pitcher, T.J. (eds). 1995. Hake: Fisheries, ecology and markets. Londres, Chapman y Hall. Fish and Fisheries Series, 15: 478 pp. Andreu, B. 1969. Las branquispinas en la caracterización de las poblaciones de Sardina pilchardus (Walb.). Inv. Pesq., 33: 425-607. Andreu, B., Anadón, E., Arté, P. y Toll, R. 1952. Sobre el significado de las variaciones de la media vertebral de la sardina de Vigo (Sardina pilchardus Walb.) estudiadas en grupos de talla de la clase 0. -

S1755267211000431jra
S1755267211000431jra Author Queries No Queries Marine Biodiversity Records, page 1 of 3. # Marine Biological Association of the United Kingdom, 2011 doi:10.1017/S1755267211000431; Vol. 00; e0; 2011 Published online 1 2 First report of Macruronus novaezelandiae 3 4 (Gadiformes, Merluccidae, Macruroninae) 5 6 7 from Atlantic tropical waters 8 1 2 3 2 9 alfredo carvalho-filho , guy marcovaldi , cla’ udio l.s. sampaio and m. isabel g. paiva 10 1Fish-Bizz Ltda, Rua Maria Garcez, 39, Sa˜o Paulo, SP, 05424-070, Brazil, 2Projeto Tamar-ICMBio, Avenida do Farol Garcia D’A´ vila, 11 s/n, Praia do Forte, Mata de Sa˜o Joa˜o, BA, 48280-000, Brazil, 3Universidade Federal de Alagoas, Unidade de Ensino Penedo, Av. Beira 12 Rio s/n, Centro Histo´rico, Penedo, AL, 57.200-000, Brazil 13 14 15 The occurrence of the merluccid Macruronus novaezelandiae from tropical waters off Bahia, eastern Brazil, is reported for the 16 first time due to the capture of an adult of 712.3 mm SL in May 2008, from a depth of 400 metres. Until then no specimen had 17 been reported north of 32829′S on the South American Atlantic coast. This new record extends the species’ range to about 18 2500 km northwards along the Brazilian coastline and is the first ever from tropical waters in the world. A comparison of 19 the morphometric characters is provided. 20 21 22 Keywords: range extension, Macruronus magellanicus, deep-sea fish, Brazil 23 24 Submitted 11 December 2010; accepted 14 March 2011 25 26 27 INTRODUCTION also observed in several other species of the family belonging 28 to the genus Merluccius, already cited above. -

Redalyc.A Review of Longnose Skates Zearaja Chilensis and Dipturus Trachyderma (Rajiformes: Rajidae)
Universitas Scientiarum ISSN: 0122-7483 [email protected] Pontificia Universidad Javeriana Colombia Vargas-Caro, Carolina; Bustamante, Carlos; Lamilla, Julio; Bennett, Michael B. A review of longnose skates Zearaja chilensis and Dipturus trachyderma (Rajiformes: Rajidae) Universitas Scientiarum, vol. 20, núm. 3, 2015, pp. 321-359 Pontificia Universidad Javeriana Bogotá, Colombia Available in: http://www.redalyc.org/articulo.oa?id=49941379004 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Univ. Sci. 2015, Vol. 20 (3): 321-359 doi: 10.11144/Javeriana.SC20-3.arol Freely available on line REVIEW ARTICLE A review of longnose skates Zearaja chilensis and Dipturus trachyderma (Rajiformes: Rajidae) Carolina Vargas-Caro1 , Carlos Bustamante1, Julio Lamilla2 , Michael B. Bennett1 Abstract Longnose skates may have a high intrinsic vulnerability among fishes due to their large body size, slow growth rates and relatively low fecundity, and their exploitation as fisheries target-species places their populations under considerable pressure. These skates are found circumglobally in subtropical and temperate coastal waters. Although longnose skates have been recorded for over 150 years in South America, the ability to assess the status of these species is still compromised by critical knowledge gaps. Based on a review of 185 publications, a comparative synthesis of the biology and ecology was conducted on two commercially important elasmobranchs in South American waters, the yellownose skate Zearaja chilensis and the roughskin skate Dipturus trachyderma; in order to examine and compare their taxonomy, distribution, fisheries, feeding habitats, reproduction, growth and longevity. -

MERLUZA AUSTRAL (Merluccius Australis)
MERLUZA AUSTRAL (Merluccius australis) por Analía R. Giussi, Susana B. García de la Rosa y Felisa Sánchez IDENTIFICACIÓN DEL RECURSO Clase: Actinopterygii. Orden: Gadiformes. Familia: Merlucciidae. Especie: Merluccius australis (Hutton, 1872). Nombre común:merluza austral, merluzón (Argentina); merluza del sur (Chile). Nombre en inglés: southern hake. Otros nombres científicos sinónimos en uso: Merluccius polylepis y Merluccius australis polylepis. DISTRIBUCIÓN GEOGRÁFICA La merluza austral es una especie que se halla ampliamente distribuida en el hemisferio sur, tanto en aguas argentinas y chilenas como en neozelandesas (Cousseau y Perrotta, 1998). En el extremo sur de América ocupa un área que se extiende, en el Océano Pacífico, al sur de los 40°S entre 50 y 600 m de pro- fundidad (Aguayo-Hernández, 1994), y en el Océano Atlántico al sur de los 50°S, desde los 100 a los 400 m de profundidad (García de la Rosa et al., 1997). Esta distribución es continua a través del Pasaje de Drake (Aguayo, 1995), hallándose individuos en la región norte del Estrecho de Magallanes (Céspedes et al., 1996). Esta especie se caracteriza por presentar hábitos demersales y está relacionada, en el Mar Argentino, con aguas frías de la Corriente de Malvinas (Otero y Simonazzi, 1980), localizándose sus ma- yores concentraciones entre 50°-55°S (García de la Rosa et al., 1997) (Figura 1). García de la Rosa et al. (1997), analizando la distribución de esta especie en el transcurso del año, observaron que en el verano las mayores concentraciones (9 y 13 t/mn2) se hallaron al sur de la Isla de los Estados (54°50'S), extendiéndose su área de distribución desde los 48° hasta 55°S y entre 100 y 200 m de profundidad. -

Duke University Dissertation Template
Fishing for Food and Fodder: The Transnational Environmental History of Humboldt Current Fisheries in Peru and Chile since 1945 by Kristin Wintersteen Department of History Duke University Date:_______________________ Approved: ___________________________ John D. French, Supervisor ___________________________ Edward Balleisen ___________________________ Jocelyn Olcott ___________________________ Gunther Peck ___________________________ Thomas Rogers ___________________________ Peter Sigal Dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of History in the Graduate School of Duke University 2011 i v ABSTRACT Fishing for Food and Fodder: The Transnational Environmental History of Humboldt Current Fisheries in Peru and Chile since 1945 by Kristin Wintersteen Department of History Duke University Date:_______________________ Approved: ___________________________ John D. French, Supervisor ___________________________ Edward Balleisen ___________________________ Jocelyn Olcott ___________________________ Gunther Peck ___________________________ Thomas Rogers ___________________________ Peter Sigal An abstract of a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of History in the Graduate School of Duke University 2011 Copyright by Kristin Wintersteen 2011 Abstract This dissertation explores the history of industrial fisheries in the Humboldt Current marine ecosystem where workers, scientists, and entrepreneurs transformed Peru and Chile into two of the top five fishing nations after World War II. As fishmeal industrialists raided the oceans for proteins to nourish chickens, hogs, and farmed fish, the global “race for fish” was marked by the clash of humanitarian goals and business interests over whether the fish should be used to ameliorate malnutrition in the developing world or extracted and their nutrients exported as mass commodities, at greater profit, as a building block for the food chain in the global North. -

Fish Stocks United Nations Food and Agriculture Organization (FAO)
General situation of world fish stocks United Nations Food and Agriculture Organization (FAO) Contents: 1. Definitions 2. Snapshot of the global situation 3. Short list of "depleted" fish stocks 4. Global list of fish stocks ranked as either "overexploited," "depleted," or recovering by region 1. Definitions Underexploited Undeveloped or new fishery. Believed to have a significant potential for expansion in total production; Moderately exploited Exploited with a low level of fishing effort. Believed to have some limited potential for expansion in total production; Fully exploited The fishery is operating at or close to an optimal yield level, with no expected room for further expansion; Overexploited The fishery is being exploited at above a level which is believed to be sustainable in the long term, with no potential room for further expansion and a higher risk of stock depletion/collapse; Depleted Catches are well below historical levels, irrespective of the amount of fishing effort exerted; Recovering Catches are again increasing after having been depleted 2. Snapshot of the global situation Of the 600 marine fish stocks monitored by FAO: 3% are underexploited 20% are moderately exploited 52% are fully exploited 17% are overexploited 7% are depleted 1% are recovering from depletion Map of world fishing statistical areas monitored by FAO Source: FAO's report "Review of the State of World Marine Fisheries Resources", tables D1-D17, ftp://ftp.fao.org/docrep/fao/007/y5852e/Y5852E23.pdf 3. Fish stocks identified by FAO as falling into its -

Stock Assessment of Hake (Merluccius Australis) on Chatham Rise for the 2019–20 Fishing Year
Stock assessment of hake (Merluccius australis) on Chatham Rise for the 2019–20 fishing year New Zealand Fisheries Assessment Report 2021/22 S.J. Holmes ISSN 1179-5352 (online) ISBN 978-1-99-100374-4 (online) April 2021 Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries websites at: http://www.mpi.govt.nz/news-and-resources/publications http://fs.fish.govt.nz go to Document library/Research reports © Crown Copyright – Fisheries New Zealand TABLE OF CONTENTS EXECUTIVE SUMMARY 1 1. INTRODUCTION 2 1.1 Description of the fishery 2 1.2 Literature review 3 2. BIOLOGY, STOCK STRUCTURE, AND ABUNDANCE INDICES 4 2.1 Biology 4 2.2 Stock structure 6 2.3 Resource surveys 6 2.4 Observer age samples 7 2.5 CPUE indices 8 3. MODEL STRUCTURE, INPUTS, AND ESTIMATION 9 3.1 Prior distributions and penalty functions 14 4. MODEL ESTIMATES FOR CHATHAM RISE HAKE 14 4.1 Developing a ‘base’ model 14 4.2 Model estimation using MCMC 21 4.3 Biomass projections 26 4.4 Management biomass targets 29 5. DISCUSSION 30 6. ACKNOWLEDGMENTS 31 7. REFERENCES 32 APPENDIX A: RESOURCE SURVEY BIOMASS INDICES FOR HAKE IN HAK 4 36 APPENDIX B: MPD FITS TO COMPOSITION DATA 38 APPENDIX C: MCMC CONVERGENCE AND DISTRIBUTION PLOTS 43 EXECUTIVE SUMMARY Holmes, S.J. (2021). Stock assessment of hake (Merluccius australis) on Chatham Rise for the 2019–20 fishing year. -
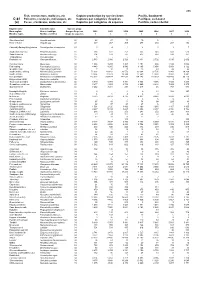
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
495 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southwest C-81 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-ouest (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoccidental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2002 2003 2004 2005 2006 2007 2008 Nombre inglés Nombre científico Grupo de especies t t t t t t t Short-finned eel Anguilla australis 22 28 27 13 10 5 ... ... River eels nei Anguilla spp 22 337 267 209 277 210 207 152 Chinook(=Spring=King)salmon Oncorhynchus tshawytscha 23 0 4 1 2 1 1 7 Southern lemon sole Pelotretis flavilatus 31 238 322 251 335 348 608 513 Sand flounders Rhombosolea spp 31 204 193 187 437 514 530 351 Tonguefishes Cynoglossidae 31 3 - - - - - - Flatfishes nei Pleuronectiformes 31 2 580 2 986 2 729 3 431 2 702 3 015 2 602 Common mora Mora moro 32 1 308 1 234 1 403 1 154 986 1 180 1 088 Red codling Pseudophycis bachus 32 4 443 8 265 9 540 8 165 5 854 5 854 6 122 Grenadier cod Tripterophycis gilchristi 32 7 10 13 13 43 29 26 Southern blue whiting Micromesistius australis 32 72 203 43 812 26 576 30 304 32 735 23 943 29 268 Southern hake Merluccius australis 32 13 834 22 623 19 344 12 560 12 858 13 892 8 881 Blue grenadier Macruronus novaezelandiae 32 215 302 209 414 147 032 134 145 119 329 103 489 96 119 Ridge scaled rattail Macrourus carinatus 32 - - - - - 9 14 Thorntooth grenadier Lepidorhynchus denticulatus 32 5 349 5 304 6 341 3 855 4 056 3 725 3 264 Grenadiers, rattails nei Macrouridae 32 3 877 4 253 3 732 2 660 2 848 7 939 8 970 Gadiformes nei Gadiformes 32 3 252 3 281 298 1 217 46 767 886 Broadgilled hagfish Eptatretus cirrhatus 33 2 - 0 0 11 508 347 Sea catfishes nei Ariidae 33 4 6 4 4 4 ..