Otolith Trace Elemental Analyses of South American Austral Hake, Merluccius Australis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ILLEGAL FISHING Which Fish Species Are at Highest Risk from Illegal and Unreported Fishing?
ILLEGAL FISHING Which fish species are at highest risk from illegal and unreported fishing? October 2015 CONTENTS EXECUTIVE SUMMARY 3 INTRODUCTION 4 METHODOLOGY 5 OVERALL FINDINGS 9 NOTES ON ESTIMATES OF IUU FISHING 13 Tunas 13 Sharks 14 The Mediterranean 14 US Imports 15 CONCLUSION 16 CITATIONS 17 OCEAN BASIN PROFILES APPENDIX 1: IUU Estimates for Species Groups and Ocean Regions APPENDIX 2: Estimates of IUU Risk for FAO Assessed Stocks APPENDIX 3: FAO Ocean Area Boundary Descriptions APPENDIX 4: 2014 U.S. Edible Imports of Wild-Caught Products APPENDIX 5: Overexploited Stocks Categorized as High Risk – U.S. Imported Products Possibly Derived from Stocks EXECUTIVE SUMMARY New analysis by World Wildlife Fund (WWF) finds that over 85 percent of global fish stocks can be considered at significant risk of Illegal, Unreported, and Unregulated (IUU) fishing. This evaluation is based on the most recent comprehensive estimates of IUU fishing and includes the worlds’ major commercial stocks or species groups, such as all those that are regularly assessed by the United Nations Food and Agriculture Organization (FAO). Based on WWF’s findings, the majority of the stocks, 54 percent, are categorized as at high risk of IUU, with an additional 32 perent judged to be at moderate risk. Of the 567 stocks that were assessed, the findings show that 485 stocks fall into these two categories. More than half of the world’s most overexploited stocks are at the highest risk of IUU fishing. Examining IUU risk by location, the WWF analysis shows that in more than one-third of the world’s ocean basins as designated by the FAO, all of these stocks were at high or moderate risk of IUU fishing. -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -

S1755267211000431jra
S1755267211000431jra Author Queries No Queries Marine Biodiversity Records, page 1 of 3. # Marine Biological Association of the United Kingdom, 2011 doi:10.1017/S1755267211000431; Vol. 00; e0; 2011 Published online 1 2 First report of Macruronus novaezelandiae 3 4 (Gadiformes, Merluccidae, Macruroninae) 5 6 7 from Atlantic tropical waters 8 1 2 3 2 9 alfredo carvalho-filho , guy marcovaldi , cla’ udio l.s. sampaio and m. isabel g. paiva 10 1Fish-Bizz Ltda, Rua Maria Garcez, 39, Sa˜o Paulo, SP, 05424-070, Brazil, 2Projeto Tamar-ICMBio, Avenida do Farol Garcia D’A´ vila, 11 s/n, Praia do Forte, Mata de Sa˜o Joa˜o, BA, 48280-000, Brazil, 3Universidade Federal de Alagoas, Unidade de Ensino Penedo, Av. Beira 12 Rio s/n, Centro Histo´rico, Penedo, AL, 57.200-000, Brazil 13 14 15 The occurrence of the merluccid Macruronus novaezelandiae from tropical waters off Bahia, eastern Brazil, is reported for the 16 first time due to the capture of an adult of 712.3 mm SL in May 2008, from a depth of 400 metres. Until then no specimen had 17 been reported north of 32829′S on the South American Atlantic coast. This new record extends the species’ range to about 18 2500 km northwards along the Brazilian coastline and is the first ever from tropical waters in the world. A comparison of 19 the morphometric characters is provided. 20 21 22 Keywords: range extension, Macruronus magellanicus, deep-sea fish, Brazil 23 24 Submitted 11 December 2010; accepted 14 March 2011 25 26 27 INTRODUCTION also observed in several other species of the family belonging 28 to the genus Merluccius, already cited above. -

MERLUZA AUSTRAL (Merluccius Australis)
MERLUZA AUSTRAL (Merluccius australis) por Analía R. Giussi, Susana B. García de la Rosa y Felisa Sánchez IDENTIFICACIÓN DEL RECURSO Clase: Actinopterygii. Orden: Gadiformes. Familia: Merlucciidae. Especie: Merluccius australis (Hutton, 1872). Nombre común:merluza austral, merluzón (Argentina); merluza del sur (Chile). Nombre en inglés: southern hake. Otros nombres científicos sinónimos en uso: Merluccius polylepis y Merluccius australis polylepis. DISTRIBUCIÓN GEOGRÁFICA La merluza austral es una especie que se halla ampliamente distribuida en el hemisferio sur, tanto en aguas argentinas y chilenas como en neozelandesas (Cousseau y Perrotta, 1998). En el extremo sur de América ocupa un área que se extiende, en el Océano Pacífico, al sur de los 40°S entre 50 y 600 m de pro- fundidad (Aguayo-Hernández, 1994), y en el Océano Atlántico al sur de los 50°S, desde los 100 a los 400 m de profundidad (García de la Rosa et al., 1997). Esta distribución es continua a través del Pasaje de Drake (Aguayo, 1995), hallándose individuos en la región norte del Estrecho de Magallanes (Céspedes et al., 1996). Esta especie se caracteriza por presentar hábitos demersales y está relacionada, en el Mar Argentino, con aguas frías de la Corriente de Malvinas (Otero y Simonazzi, 1980), localizándose sus ma- yores concentraciones entre 50°-55°S (García de la Rosa et al., 1997) (Figura 1). García de la Rosa et al. (1997), analizando la distribución de esta especie en el transcurso del año, observaron que en el verano las mayores concentraciones (9 y 13 t/mn2) se hallaron al sur de la Isla de los Estados (54°50'S), extendiéndose su área de distribución desde los 48° hasta 55°S y entre 100 y 200 m de profundidad. -

Fish Stocks United Nations Food and Agriculture Organization (FAO)
General situation of world fish stocks United Nations Food and Agriculture Organization (FAO) Contents: 1. Definitions 2. Snapshot of the global situation 3. Short list of "depleted" fish stocks 4. Global list of fish stocks ranked as either "overexploited," "depleted," or recovering by region 1. Definitions Underexploited Undeveloped or new fishery. Believed to have a significant potential for expansion in total production; Moderately exploited Exploited with a low level of fishing effort. Believed to have some limited potential for expansion in total production; Fully exploited The fishery is operating at or close to an optimal yield level, with no expected room for further expansion; Overexploited The fishery is being exploited at above a level which is believed to be sustainable in the long term, with no potential room for further expansion and a higher risk of stock depletion/collapse; Depleted Catches are well below historical levels, irrespective of the amount of fishing effort exerted; Recovering Catches are again increasing after having been depleted 2. Snapshot of the global situation Of the 600 marine fish stocks monitored by FAO: 3% are underexploited 20% are moderately exploited 52% are fully exploited 17% are overexploited 7% are depleted 1% are recovering from depletion Map of world fishing statistical areas monitored by FAO Source: FAO's report "Review of the State of World Marine Fisheries Resources", tables D1-D17, ftp://ftp.fao.org/docrep/fao/007/y5852e/Y5852E23.pdf 3. Fish stocks identified by FAO as falling into its -

Description of Merluccius Tasmanicus Sp. Nov. and Redescription
J. Mar. Biol. Ass. U.K. (2006), 86,193^199 Printed in the United Kingdom Description of Merluccius tasmanicus sp.nov.and redescription of Merluccius australis (Pisces: Merlucciidae) P O J. Matallanas* and D. Lloris *Unidad de Zoolog|¤a, Departamento de Biolog|¤a Animal, Biolog|¤aVegetal y Ecolog|¤a. Universidad Auto¤noma de Barcelona, 08193, O Bellaterra, Barcelona, Spain. Institut de Cie' ncies del Mar (CMIMA-CSIC), Passeig Mar|¤tim de la Barceloneta 37^49, P 08003 Barcelona, Spain. E-mail: [email protected]. Corresponding author, e-mail: [email protected] A new hake species, Merluccius tasmanicus sp. nov., is described from New Zealand waters and another species, Merluccius australis is redescribed. Merluccius tasmanicus sp. nov. di¡ers from all other congeneric species in the following combination of characters: upper pro¢le of the head slowly concave; lateral line slowly concave in the caudal region; body depth 4.9^5.9 times in standard length (SL); orbital diameter 6.1^7.1 times in head length, 2.1^2.2 times in snout length and 1.6^1.9 times in interorbital width; second dorsal ¢n rays, 42^43; anal ¢n rays, 42^44; lateral line scales *164. Merluccius australis is redescribed to clarify the identity of this species. Merluccius australis di¡ers from all other congeneric species in the following combination of characters: upper pro¢le of the head straight; lateral line straight in the caudal region; body depth 6.6^7.1times in SL; orbital diameter 4.5^5.4 times in head length, 1.2^1.7 times in snout length and 1.0^1.3 times in interorbital width; second dorsal ¢n rays, 40^43; anal ¢n rays, 40^43; lateral line scales, more than 155. -

Stock Assessment of Hake (Merluccius Australis) on Chatham Rise for the 2019–20 Fishing Year
Stock assessment of hake (Merluccius australis) on Chatham Rise for the 2019–20 fishing year New Zealand Fisheries Assessment Report 2021/22 S.J. Holmes ISSN 1179-5352 (online) ISBN 978-1-99-100374-4 (online) April 2021 Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries websites at: http://www.mpi.govt.nz/news-and-resources/publications http://fs.fish.govt.nz go to Document library/Research reports © Crown Copyright – Fisheries New Zealand TABLE OF CONTENTS EXECUTIVE SUMMARY 1 1. INTRODUCTION 2 1.1 Description of the fishery 2 1.2 Literature review 3 2. BIOLOGY, STOCK STRUCTURE, AND ABUNDANCE INDICES 4 2.1 Biology 4 2.2 Stock structure 6 2.3 Resource surveys 6 2.4 Observer age samples 7 2.5 CPUE indices 8 3. MODEL STRUCTURE, INPUTS, AND ESTIMATION 9 3.1 Prior distributions and penalty functions 14 4. MODEL ESTIMATES FOR CHATHAM RISE HAKE 14 4.1 Developing a ‘base’ model 14 4.2 Model estimation using MCMC 21 4.3 Biomass projections 26 4.4 Management biomass targets 29 5. DISCUSSION 30 6. ACKNOWLEDGMENTS 31 7. REFERENCES 32 APPENDIX A: RESOURCE SURVEY BIOMASS INDICES FOR HAKE IN HAK 4 36 APPENDIX B: MPD FITS TO COMPOSITION DATA 38 APPENDIX C: MCMC CONVERGENCE AND DISTRIBUTION PLOTS 43 EXECUTIVE SUMMARY Holmes, S.J. (2021). Stock assessment of hake (Merluccius australis) on Chatham Rise for the 2019–20 fishing year. -
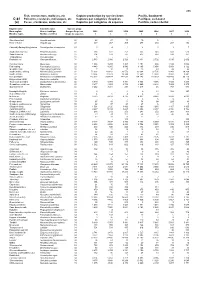
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
495 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southwest C-81 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-ouest (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoccidental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2002 2003 2004 2005 2006 2007 2008 Nombre inglés Nombre científico Grupo de especies t t t t t t t Short-finned eel Anguilla australis 22 28 27 13 10 5 ... ... River eels nei Anguilla spp 22 337 267 209 277 210 207 152 Chinook(=Spring=King)salmon Oncorhynchus tshawytscha 23 0 4 1 2 1 1 7 Southern lemon sole Pelotretis flavilatus 31 238 322 251 335 348 608 513 Sand flounders Rhombosolea spp 31 204 193 187 437 514 530 351 Tonguefishes Cynoglossidae 31 3 - - - - - - Flatfishes nei Pleuronectiformes 31 2 580 2 986 2 729 3 431 2 702 3 015 2 602 Common mora Mora moro 32 1 308 1 234 1 403 1 154 986 1 180 1 088 Red codling Pseudophycis bachus 32 4 443 8 265 9 540 8 165 5 854 5 854 6 122 Grenadier cod Tripterophycis gilchristi 32 7 10 13 13 43 29 26 Southern blue whiting Micromesistius australis 32 72 203 43 812 26 576 30 304 32 735 23 943 29 268 Southern hake Merluccius australis 32 13 834 22 623 19 344 12 560 12 858 13 892 8 881 Blue grenadier Macruronus novaezelandiae 32 215 302 209 414 147 032 134 145 119 329 103 489 96 119 Ridge scaled rattail Macrourus carinatus 32 - - - - - 9 14 Thorntooth grenadier Lepidorhynchus denticulatus 32 5 349 5 304 6 341 3 855 4 056 3 725 3 264 Grenadiers, rattails nei Macrouridae 32 3 877 4 253 3 732 2 660 2 848 7 939 8 970 Gadiformes nei Gadiformes 32 3 252 3 281 298 1 217 46 767 886 Broadgilled hagfish Eptatretus cirrhatus 33 2 - 0 0 11 508 347 Sea catfishes nei Ariidae 33 4 6 4 4 4 .. -

ARGENTINE PATAGONIAN TOOTHFISH FISHERY (Dissostichus Eleginoides)
Assessment against MSC Principles and Criteria for: ARGENTINE PATAGONIAN TOOTHFISH FISHERY (Dissostichus eleginoides) 27th June, 2014 AUTHORS: Hanchet, S., Morsan, E., Bridi, R. and C. Medina Foucher (OIA Technical) CLIENT GROUP: Argenova S.A., Empresa Pesquera de la Patagonia y Antártida S.A. (PESANTAR S.A.), Estremar S.A. and San Arawa S.A. PUBLIC COMMENT DRAFT REPORT Assessment against MSC Principles and Criteria for ARGENTINE PATAGONIAN TOOTHFISH FISHERY (Dissostichus eleginoides) 27th June, 2014 CLIENT GROUP: Argenova S.A. Contact: Jaime Pérez Pena Address: Av. Belgrano 920. (1092) Buenos Aires, Argentina Tel: +54 11 5217 7830 Empresa Pesquera de la Patagonia y Antártida S.A. (PESANTAR S.A.) Contact: Daniel Rivera Address: Carlos Pellegrini 855 Floor 9. (1009) Buenos Aires, Argentina Tel: +54 11 4325 9553 Estremar S.A Contact: Marcelo González Address: Av. Olazábal 1515 Floor 11 Of. 1102-1103. (1428) Buenos Aires, Argentina Tel: +54 11 4343 2673 San Arawa S.A. Contact: Eduardo González Lemmi Address: 25 de Mayo 260 Floor 1 Of. 4. (9410) Ushuaia, Tierra del Fuego Tel: +54 223 492 2215 CERTIFICATION BODY: OrganiZación Internacional Agropecuaria S.A. (OIA) Address: Av. Santa Fe 830, Acassuso (B1641ABN), Buenos Aires, Argentina Tel/Fax: (+54) 11 4793-4340 [email protected] ∙ www.oia.com.ar File: APT – Public Comment Draft Reportsssssssssssssssssssssssssssssssssssssssssssssssssssssssssssssss 1 Date of issue: 27/06/2014 Table of Contents. Glossary .................................................................................................................................................. -

Phylogenetic Prospecting for Cryptic Species of the Genus Merluccius
www.nature.com/scientificreports OPEN Phylogenetic prospecting for cryptic species of the genus Merluccius (Actinopterygii: Merlucciidae) Montse Pérez1, María Fernández‑Míguez1,2, Jesús Matallanas3, Domingo Lloris4 & Pablo Presa2* Hakes of the genus Merluccius include 11 valid species as well a number of rare morphotypes suspected to be “cryptic species”. Concatenated nucDNA ITS1‑rDNA and mtDNA cyt b sequences plus nested ITS1Nes sequences allowed to ascribe 14 specimens of nine rare morphotypes from the South Pacifc and the South Atlantic to the phylogenetic backbone of this genus. Bayesian analyses pointed to M. bilinearis and M. albidus as the oldest species of the genus and the New World cluster, respectively. The phylogenetic status of M. angustimanus from the upper Gulf of California suggests its hybrid origin between M. gayi and M. productus from about 0.25 MYA, although an ever since confnement of a subset of those species cannot be ruled out. The molecular phylodiagnostic test suggests a common origin of all rare morphotypes and the absence of cryptic hake species in the Southern Cone. The molecular background of the morphotypes distributed between the Western Pacifc South of New Zealand and the western Atlantic South of Argentina is compatible with their hybrid origin between M. gayi and both, M. australis or M. hubbsi, respectively. Te genus Merluccius comprises 11 valid species that occur on most temperate and tropical continental shelves except the Asian shores of the Pacifc Ocean1. Hakes show an anti-tropical distribution in the Atlantic Ocean and the Eastern Pacifc and a latitudinal bathymetric overlap between isotherms 7 °C and 23 °C2–4. -

PROYECTO FIP Nº 2007-29 (2ª Licitación)
PONTIFICIA UNIVERSIDAD CATOLICA DE VALPARAÍSO FACULTAD DE RECURSOS NATURALES ESCUELA DE CIENCIAS DEL MAR Casilla 1020, Valparaíso, Chile. PROYECTO FIP Nº 2007-29 (2ª Licitación) BASES TÉCNICAS PARA EL PLAN DE MANEJO DE LA PESQUERÍA DEMERSAL AUSTRAL INFORME DE FINAL Valparaíso, Agosto de 2009 i TITULO DEL PROYECTO : “Bases técnicas para el plan de manejo de la pesquería demersal austral” REQUIRENTE : Consejo del Fondo de Investigación Pesquera Proyecto FIP Nº 2007-29 (2ª Licitación) CONTRAPARTE : Pontificia Universidad Católica de Valparaíso Facultad de Recursos Naturales UNIDAD EJECUTORA : Escuela de Ciencias del Mar Av. Altamirano 1480 Casilla 1020 Valparaíso JEFE DE PROYECTO : René Cerda D’Amico Escuela de Ciencias del Mar Fono (56) (32) 274249 Fax (56) (32) 274206 E-mail: [email protected] ii EQUIPO DE TRABAJO INVESTIGADOR INSTITUCIÓN René Cerda D’Amico Pontificia Universidad Católica de Valparaíso – PUCV Guillermo Martínez Pontificia Universidad Católica de Valparaíso – PUCV González Eleuterio Yánez Rodríguez Pontificia Universidad Católica de Valparaíso – PUCV Exequiel González Poblete Pontificia Universidad Católica de Valparaíso – PUCV Patricio Arana Espina Pontificia Universidad Católica de Valparaíso – PUCV Héctor Trujillo Portales Pontificia Universidad Católica de Valparaíso – PUCV Héctor Luis Morales Zabala Consultor Independiente José Iván Sepúlveda Vidal Pontificia Universidad Católica de Valparaíso – PUCV ESPECIALISTAS INSTITUCIÓN Bernardo Zapata N. Consultor Independiente Luis Villegas C. Consultor Independiente CO-INVESTIGADORES INSTITUCIÓN COLABORADORES Jaime Aguilera Pontificia Universidad Católica de Valparaíso – PUCV Lorena Álvarez Pontificia Universidad Católica de Valparaíso – PUCV Claudia Jiménez Pontificia Universidad Católica de Valparaíso – PUCV Andrea Bello S. Pontificia Universidad Católica de Valparaíso – PUCV Pedro Romero Pontificia Universidad Católica de Valparaíso – PUCV Dante Queirolo Pontificia Universidad Católica de Valparaíso – PUCV Ivonne Montenegro U. -

HAKES of the WORLD (Family Merlucciidae)
ISSN 1020-8682 FAO Species Catalogue for Fishery Purposes No. 2 HAKES OF THE WORLD (Family Merlucciidae) AN ANNOTATED AND ILLUSTRATED CATALOGUE OF HAKE SPECIES KNOWN TO DATE (Family MERLUCCIIDAE) FAO Species Catalogue for Fishery Purposes No. 2 FIR/Cat. 2 HAKES OF THE WORLD (Family Merlucciidae) AN ANNOTATED AND ILLUSTRATED CATALOGUE OF HAKE SPECIES KNOWN TO DATE by D. Lloris Instituto de Ciencias del Mar (CMIMA-CSIC) Barcelona, Spain J. Matallanas Facultad de Ciencias Universidad Autónoma de Barcelona Bellaterra, Barcelona, Spain and P. Oliver Instituto Español de Oceanografia Palma de Mallorca, Spain FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2005 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. ISBN 92-5-104984-X All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of material in this information product for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission should be addressed to the Chief, Publishing Management Service, Information Division, FAO, Viale delle Terme di Caracalla, 00100 Rome, Italy by e-mail to [email protected] © FAO 2005 Hakes of the World iii PREPARATION OF THIS DOCUMENT his catalogue was prepared under the FAO Fisheries Department Regular Programme by the Species Identification and TData Programme in the Marine Resources Service of the Fishery Resources Division.