Antistreptolysin O Screen Sunquest Code:ASOS Place of Service:Labcorp Caromont LAB530 17 Hydroxprogesteron
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DIAGMOL Town, ZIPCODE :…………………………………………………… Director : Prof
Mr. Mrs. (IN UPPER CASE, please) Name:……………………………………………………. Maiden Name:……………………………………. DMGL / Service de Médecine Génétique Centre d’accueil des prélèvements (CAP) First name :………………………………………………… Bâtiment des Laboratoires (BATLab), local 8D-0-850.1 Date of birth : ............ / ........... / …………… 4 rue Gabrielle-Perret-Gentil, 1211 Genève 14 Legal representative (for minors) : father mother Molecular and Genomic Diagnostics Laboratory Name/first name :…………………………………………… Street/N°:……………………………………………………… http://www.hug-ge.ch/feuilles-de-demande DIAGMOL Town, ZIPCODE :…………………………………………………… Director : Prof. Stylianos ANTONARAKIS Hospitalisation Unit: …………… Physician :…………………… Lab managers: N° EdS : …………………………………………………………… Dr J.-L. BLOUIN, Dr Th. NOUSPIKEL, Dr. M. GUIPPONI Invoice address: Patient Prescriptor Insurance [email protected], [email protected], [email protected] Type of case : Disease AI Accident Pregnancy Lab direct or results: Phone/FAX: +41 (0) 22 37 21 826 / 21 860 N° AVS (Mandatory for AI) : ………………………...................... Sample Entrance Center (CAP) : Phone +41 (0) 22 37 21 800 Insurance : ………………… Insured N° : ……………………… PHYSICIAN PHYSICIAN (NAME/First name - Street/N°- Town, ZIPCODE - Phone/FAX. IN UPPER CASES, PLEASE) COPY TO OTHER PHYSICIAN (NAME/First name - Street/N°- Town, ZIPCODE - Phone/FAX. IN UPPER CASES, PLEASE) « The laboratory is granted permission by the Physician/Patient to transmit copies of the report to other physicians» Opposition of the patient to the registration of this request results in the electronic patient record (DPI) of the HUG If the patient belongs to a family already known to the laboratory, please indicate index case NAME: CLINICAL INFORMATIONS given by the physician: Ethnic origins Father Mother Currently pregnant Date of last menses Number of weeks of amenorrhea SAMPLE(S) Most of our tests work from 4 ml of blood in EDTA (children <2 ans : 1 On every and single NAME First name ml : ok) or from purified DNA (some exceptions apply for some tests) tube. -

Metabolism of Reverse Triiodothyronine by Isolated Rat Hepatocytes
Metabolism of reverse triiodothyronine by isolated rat hepatocytes. S J Rooda, … , M A van Loon, T J Visser J Clin Invest. 1987;79(6):1740-1748. https://doi.org/10.1172/JCI113014. Research Article Reverse triiodothyronine (rT3) is metabolized predominantly by outer ring deiodination to 3,3'-diiodothyronine (3,3'-T2) in the liver. Metabolism of rT3 and 3,3'-T2 by isolated rat hepatocytes was analyzed by Sephadex LH-20 chromatography, high performance liquid chromatography, and radioimmunoassay, with closely agreeing results. Deiodinase activity was inhibited with propylthiouracil (PTU) and sulfotransferase activity by sulfate depletion or addition of salicylamide or dichloronitrophenol. Normally, little 3,3'-T2 production from rT3 was observed, and 125I- was the main product of both 3, [3'-125I]T2 and [3',5'-125I]rT3. PTU inhibited rT3 metabolism but did not affect 3,3'-T2 clearance as explained by accumulation of 3,3'-T2 sulfate. Inhibition of sulfation did not affect rT3 clearance but 3,3'-T2 metabolism was greatly diminished. The decrease in I- formation from rT3 was compensated by an increased recovery of 3,3'-T2 up to 70% of rT3 metabolized. In conclusion, significant production of 3,3'-T2 from rT3 by rat hepatocytes is only observed if further sulfation is inhibited. Find the latest version: https://jci.me/113014/pdf Metabolism of Reverse Triiodothyronine by Isolated Rat Hepatocytes Sebo Jan Eelkman Rooda, Maria A. C. van Loon, and Thoo J. Vissef Department ofInternal Medicine III and Clinical Endocrinology, Erasmus University Medical School, Rotterdam, The Netherlands Abstract It deiodinates only the outer ring of substrates such as T4 and rT3 (2-4). -

United States Patent (10) Patent N0.: US 7,288,257 B2 Powell (45) Date of Patent: Oct
US007288257B2 (12) United States Patent (10) Patent N0.: US 7,288,257 B2 Powell (45) Date of Patent: Oct. 30, 2007 (54) DIAGNOSIS AND TREATMENT OF HUMAN 5,342,788 A 8/1994 Kunst et a1. .............. .. 436/500 DORMANCY SYNDROME 5,691,456 A 11/1997 AdamcZyk et al. ....... .. 530/405 6,087,090 A 7/2000 Mascarenhas ................ .. 435/4 (76) Inventor: Michael Powell, 150 Catherine Lance, 2003/0007941 A1 1/2003 Cornelius et a1, Suite 1, Grass Valley, CA (US) 95945 ( * ) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 OTHER PUBLICATIONS U.S.C. 154(b) by 199 days. _ Hannah V. Carey, The American Physiological Society, Physical _ Rev 83; Mammalian Hibernation: Cellular and Molecular (21) Appl' NO" 10/444’845 Responses to Depressed Metabolism and Low Temperature, 2003, (22) Filed: May 23, 2003 PP' 1153'1181' _ _ _ Primary ExamineriRuth A Davis (65) Pnor Pubhcatlon Data (74) Attorney, Agent, or F irmiBorson LaW Group, PC; D. US 2003/0228628 A1 Dec. 11, 2003 Benjamin Borson Related US. Application Data (57) ABSTRACT (60) Provisional application No. 60/382,913, ?led on May $112002’ provlslonal apphcanon NO‘ 60/383’271’ New methods for diagnosis of human dormancy syndrome e on May 24’ 2002' are provided. Human dormancy syndrome is characterized (51) Int C1 by elevated serum ratio of rT3/iT 3 compared to a population 1462K 3'9/00 (2006 01) of normal subjects from Which subjects suffering from A 61K 38/22 (200601) ?bromyalgia, chronic fatigue, obesity, dementias including A 61K 38/27 (200601) AlZheimer’s Disease and related dormancy conditions are C1 2 Q 1/00 (200601) excluded, and the presence of one or more ?ndings related ' A / A / A to reduced activity including torpor, chronic fatigue, insulin (52) US. -

Comprehensive Thyroid Plus Adrenal Report
Comprehensive Thyroid Plus Adrenal Report Jane Doe SAMPLEDate Collected: 2/13/2017 Comprehensive Thyroid Plus Adrenal Report Patient Name: Doe, Jane Batch Number: B0000 Patient DOB: 12/10/1960 Accession Number: Q00000 Gender: F Date Received: 2/14/2017 Physician Jon Doe, ND Report Date: 2/22/2017 Test Patient Results Reference Value DHEAS µg/dL 0 125 250 375 500 107 35 - 430 TSH µIU/mL 0.020 1.260 2.500 3.740 4.980+ 7.030 0.358 - 3.74 T4, Total µg/dL 0.5 4.4 8.3 12.1 16.0 7.2 4.5 - 12.5 T3, Free pg/mL 0.5 1.9 3.3 4.6 6.0 3.6 2.2 - 4.0 T4, Free ng/dL 0.10 0.83 1.55 2.28 3.00 0.87 0.76 - 1.46 Cortisol µg/dL 0.2 11.4 22.6 33.8 45.0 6.5 3.1 - 22.4 AntiTPO Ab IU/mL 10.0 20.0 30.0 40.0 50.0 + > 1000.0 0.0 - 35.0 AntiThyroglobulin Ab IU/mL 20 30 40 50 60 + 97 ND - 40 Thyroglobulin ng/mL 0 20 40 60 80 38 <= 55 Thyroxinebinding globulin, TBG µg/mL SAMPLE0 11 23 34 45 20 14 - 31 10401 Town Park Drive, Houston, Texas 77072 USA CLIA# 45D0710715 (800)227-LABS(5227) / (713)-621-3101 James W. Jacobson, Ph.D., Laboratory Director Comprehensive Thyroid Plus Adrenal Report Patient Name: Doe, Jane Batch Number: B0000 Patient DOB: 12/10/1960 Accession Number: Q00000 Gender: F Date Received: 2/14/2017 Physician Jon Doe, ND Report Date: 2/22/2017 Test Component Flag Result Reference Range DHEA-S µg/dL 107 35 - 430 TSH µIU/mL H 7.030 0.358 - 3.74 T4, Total µg/dL 7.2 4.5 - 12.5 T3, Free pg/mL 3.6 2.2 - 4.0 T4, Free ng/dL 0.87 0.76 - 1.46 Cortisol µg/dL 6.5 3.1 - 22.4 Anti-TPO Ab IU/mL H > 1000.0 0.0 - 35.0 Anti-Thyroglobulin Ab IU/mL H 97 ND - 40 Thyroglobulin ng/mL 38 <= 55 Thyroxine-binding globulin, TBGSAMPLE µg/mL 20 14 - 31 10401 Town Park Drive, Houston, Texas 77072 USA CLIA# 45D0710715 (800)227-LABS(5227) / (713)-621-3101 James W. -

Chang Et Al Thyroid
Available online at www.sciencedirect.com Toxicology 243 (2008) 330–339 Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (PFOS)ଝ Shu-Ching Chang a, Julie R. Thibodeaux b,1, Mary L. Eastvold c, David J. Ehresman a, James A. Bjork d, John W. Froehlich e,2, Christopher Lau b, Ravinder J. Singh c, Kendall B. Wallace d, John L. Butenhoff a,∗ a Medical Department, 3M Company, St. Paul, MN 55144, United States b United States Environmental Protection Agency, ORD, NHEERL, Reproductive Toxicology Division, Research Triangle Park, NC 27711, United States c Mayo Clinic and Foundation, Department of Laboratory Medicine and Pathology, Rochester, MN 55095, United States d University of Minnesota, Medical School, Department of Biochemistry and Molecular Biology, Duluth, MN 55812, United States e Pace Analytical Services, Inc., Minneapolis, MN 55414, United States Received 29 August 2007; received in revised form 18 October 2007; accepted 20 October 2007 Available online 26 October 2007 Abstract Introduction: Perfluorooctanesulfonate (PFOS) is widely distributed and persistent in humans and wildlife. Prior toxicological studies have reported decreased total and free thyroid hormones in serum without a major compensatory rise in thyrotropin (TSH) or altered thyroid gland histology. Although these animals (rats, mice and monkeys) might have maintained an euthyroid state, the basis for hypothyroxinemia remained unclear. We undertook this study to investigate the causes for the PFOS-induced reduction of serum total thyroxine (TT4) in rats. Hypotheses: We hypothesized that exposure to PFOS may increase free thyroxine (FT4) in the rat serum due to the ability of PFOS to compete with thyroxine for binding proteins. -

Ontogenesis of Iodothyronine-5'-Deiodinase
Ontogenesis of iodothyronine-5'-deiodinase. Induction of 5'- deiodinating activity by insulin, glucocorticoid, and thyroxine in cultured fetal mouse liver. K Sato, … , T Tsushima, K Shizume J Clin Invest. 1984;74(6):2254-2262. https://doi.org/10.1172/JCI111652. Research Article To elucidate the regulatory mechanism of ontogenetic development of iodothyronine-5'-deiodinase in the fetal and neonatal period, fetal mouse liver of the 19th day of gestation, in which no iodothyronine-5'-deiodinating activity was detectable, was cultured in Dulbecco-Vogt medium supplemented with 10% thyroid hormone-depleted fetal calf serum, insulin, hydrocortisone, and thyroid hormones. Iodothyronine-5'-deiodinating activity of the homogenate was assessed by the amount of iodide released from outer-ring-labeled reverse T3 and expressed as picomoles of 127I- per milligram of protein per minute. The enzyme activity was induced in a dose-dependent manner; optimal concentrations for insulin, hydrocortisone, and thyroxine were 1 microgram/ml, 0.4 microgram/ml, and 10(-6) M, respectively. Without supplementation of either hydrocortisone or thyroxine, no 5'-deiodination was detected. The enzyme activity was observed after 3 d of culture, peaked at days 14-20, and then gradually decreased. Lineweaver-Burk analysis revealed that the increase in activity was primarily due to an increase in Vmax (day 3, 0.2 pmol/mg protein per min; day 20, 2.5 pmol/mg protein per min). Half maximal thyroxine (T4) and triiodothyronine (T3) concentrations were 1 X 10(-7) M (free T4: 4 X 10(-10) M), and 2 X 10(-9) M (free T3: 5.0 X 10(-11) M), respectively, whereas reverse T3 did not elicit any activity at 10(-8)-10(-6) M. -

UNSCEAR 2001 Report to the General Assembly, with Scientific Annex
HEREDITARY EFFECTS OF RADIATION United Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR 2001 Report to the General Assembly, with Scientific Annex UNITED NATIONS HEREDITARY EFFECTS OF RADIATION United Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR 2001 Report to the General Assembly, with Scientific Annex UNITED NATIONS New York, 2001 NOTE The report of the Committee without its scientific annex appears as Official Records of the General Assembly. Fifty-sixth Session, Supplement No. 46 (N56146). The designation employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the pad of the Secretariat of the United Nationsconcerning the legal status of any country, temtory, city orarea, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The country names used in this document are, In most cases, those that were in use at the time the data were collected or the text prepared. In othercases, however, the names have been updated, where this was posslble and appropriate, to reflect political changes. UNITED NATIONS PUBLICATION Sales No. E.O1 .IX.2 ISBN 92-1-1 42244-2 Report of the United Nations Scientific Committee on the Effects of Atomic Radiation to the General Assembly 1. During the past few years, the United Nations 4. The Committee wishes to acknowledge the assistance Scientific Committee on the Effects of Atomic Radiation1 of the consultant, K. Sankaranarayanan, in the preparation has undertaken broad reviews of the sources and effects of of the scientific annex and the advice of the international ionizing radiation. -

Uncommon Forms of Diabetes
Clinical Medicine 2021 Vol 21, No 4: e337–41 CME: DIABETES Uncommon forms of diabetes Authors: Yun-Ni LeeA and Mohammed SB HudaB Diabetes mellitus is a common condition which all clinicians and insulin independence. It is estimated to account for 1%–2% will encounter in their clinical practice. The most common of patients diagnosed with diabetes and, in the UK, the prevalence form is type 2 diabetes followed by type 1 diabetes. However, of MODY is estimated to be at 108 cases per million.3 However, there are many other atypical forms of diabetes which are it may be a significant underestimate and these figures are not important for a clinician to consider as it can impact on the accurate until large population screening studies are performed. ABSTRACT diagnosis and their management. The most common mutations are hepatocyte nuclear factor-1- This article focuses on maturity onset diabetes of the young alpha (HNF1α; 52%), glucokinase (GCK; 32%) and HNF4α (10%), (MODY), latent autoimmune diabetes in adults (LADA), see Table 2.3 ketosis-prone diabetes and other secondary forms of diabetes such as pancreatic cancer and haemochromatosis. We briefly Hepatocyte nuclear factor-1-alpha gene describe the key clinical features of these forms of diabetes and their investigations and treatment. Formerly called MODY3, mutations on the HNF1α gene on chromosome 3 are associated with a progressive defect of insulin secretion.4 Mutations here also result in low renal threshold for 5 Introduction glucose and thus mutation carriers have detectable glycosuria. In the UK, around 90% of people with diabetes have type 2 diabetes (T2D), around 8% have type 1 diabetes (T1D) and around 2% have other forms of diabetes.1 Key points Typically, we see T1D present in a young, lean patient with Suspect other uncommon forms of diabetes if the clinical marked symptoms of polyuria, polydipsia, weight loss and diabetic picture does not fit type 1 or type 2 diabetes. -

Dissertation / Doctoral Thesis
DISSERTATION / DOCTORAL THESIS Titel der Dissertation /Title of the Doctoral Thesis „ The natural compound curcumin: impact of cellular uptake and metabolism on in vitro activity “ verfasst von / submitted by Qurratul Ain Jamil angestrebter akademischer Grad / in partial fulfilment of the requirements for the degree of Doktorin der Naturwissenschaften (Dr.rer.nat.) Wien, 2018 / Vienna 2018 Studienkennzahl lt. Studienblatt / A 796 610 449 degree programme code as it appears on the student record sheet: Dissertationsgebiet lt. Studienblatt / Pharmazie, Klinische Pharmazie und Diagnostik field of study as it appears on the student record sheet: Pharmacy, Clinical Pharmacy and Diagnostics Betreut von / Supervisor: Ao.Univ.Prof.Magpharm.Dr.rer.nat.WalterJӓger “Verily in the Creation of the Heavens and the Earth, and in the Alteration of Night and Day, and the Ships which Sail through the Sea with that which is of used to Mankind, and the Water (Rain) which God sends down from the Sky and makes Earth Alive there with after its Death, and the moving Creatures of all kind that he has Scattered therein, and the Veering of Winds and Clouds which are held between the Sky and the Earth, are indeed Signs for Peoples of Understanding.” Acknowledgements While writing this section, I remember the first day in my lab, having a warm welcome from my supervisor; ao. Univ.-Prof. Mag. Dr. Walter Jӓger (Division of Clinical Pharmacy and Diagnostics, University of Vienna). He was waiting for me, offered me coffee and told me how to operate the coffee machine. He showed me, my office and asked me to make a list of all stuff, I need for daily work. -

Mat Kadi Tora Tutti O Al Ut Hit Hitta Atuh
MAT KADI TORA TUTTI USO AL20180235194A1 UT HIT HITTA ATUH ( 19) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2018 /0235194 A1 Fahrenkrug et al. (43 ) Pub . Date : Aug . 23, 2018 ( 54 ) MULTIPLEX GENE EDITING Publication Classification (51 ) Int. Ci. ( 71 ) Applicant: Recombinetics , Inc ., Saint Paul, MN A01K 67/ 027 (2006 . 01 ) (US ) C12N 15 / 90 ( 2006 .01 ) (72 ) Inventors : Scott C . Fahrenkrug, Minneapolis , (52 ) U . S . CI. MN (US ) ; Daniel F . Carlson , CPC .. .. A01K 67 / 0276 (2013 . 01 ) ; C12N 15 / 907 Woodbury , MN (US ) ( 2013 .01 ) ; A01K 67 /0275 ( 2013 .01 ) ; A01K 2267/ 02 (2013 .01 ) ; AOIK 2217 / 15 (2013 .01 ) ; AOIK 2227 / 108 ( 2013 .01 ) ; AOIK 2217 /07 (21 ) Appl. No. : 15 /923 , 951 ( 2013 .01 ) ; A01K 2227/ 101 (2013 .01 ) ; AOIK ( 22 ) Filed : Mar. 16 , 2018 2217 /075 ( 2013 .01 ) (57 ) ABSTRACT Related U . S . Application Data Materials and methods for making multiplex gene edits in (62 ) Division of application No . 14 /698 ,561 , filed on Apr. cells and are presented . Further methods include animals 28 , 2015, now abandoned . and methods of making the same . Methods of making ( 60 ) Provisional application No . 61/ 985, 327, filed on Apr. chimeric animals are presented , as well as chimeric animals . 28 , 2014 . Specification includes a Sequence Listing . Patent Application Publication Aug . 23 , 2018 Sheet 1 of 13 US 2018 / 0235194 A1 GENERATION OF HOMOZYGOUS CATTLE EDITED AT ONE ALLELE USING SINGLE EDITS Edit allele , Raise FO to Mate FO enough times Raise F1s to Mate F1 siblings Clone cell , sexual maturity , to produce enough F1 sexualmaturity , to make Implant, Gestate 2 years generation carrying 2 years homozygous KO , 9 months , birth edited allele to mate 9 months of FO with each other Generation Primary Fibroblasts ? ? ? Time, years FIG . -
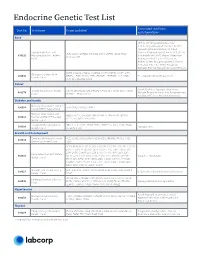
Endocrine Genetic Test List
Endocrine Genetic Test List Associated conditions Test No. Test Name Genes Included and phenotypes Bone HPP, X-Linked Hypophosphatemia, X-Linked Hypophosphatemic Rickets, XLH, Hypophosphatemic Rickets, X-Linked Hypophosphatasia and Dominant Hypophosphatemic Rickets (XLHR), ALPL, CLCN5, CYP2R1, CYP27B1, DMP1, ENPP1, FGF23, PHEX, 630292 Hypophosphatemic Rickets X-Linked Rickets (XLR), Vitamin D-Resistant SLC34A3, VDR Panel Rickets, X-Linked Vitamin D-Resistant Rickets (VDRR), Hypophosphatemic Vitamin D-Resistant Rickets (HPDR), Phosphate Diabetes, Familial Hypophosphatemic Rickets BMP1, COL1A1, COL1A2, CREB3L1, CRTAP, FKBP10, IFITM5, LRP5, Osteogenesis Imperfecta 630543 MBTPS2, P3H1, PLOD2, PPIB, SERPINF1, SERPINH1, SP7, SPARC, OI, Juvenile Primary Osteoporosis Genetic Panel TENT5A, TMEM38B, WNT1 Cancer Familial Isolated Hyperparathyroidism, VistaSeq® Endocrine Cancer CDC73, MAX, MEN1, NF1, PRKAR1A, PTEN, RET, SDHB, SDHC, SDHD, 481374 Multiple Endocrine Neoplasia, Paraganglioma, Panel* TMEM127, TP53 and VHL Parathyroid Cancer, Pheochromocytoma Diabetes and Insulin Maturity-Onset Diabetes of the 630568 GCK, HNF1A, HNF1B, HNF4A Young (MODY) 4-gene Panel Maturity-Onset Diabetes of ABCC8, APPL1, BLK, GCK, HNF1A, HNF1B, HNF4A, INS, KCNJ11, 630513 the Young (MODY) Expanded KLF11, NEUROD1, PAX4, PDX1 Genetic Panel Congenital Hyperinsulinism ABCC8, GCK, GLUD1, HADH, HNF1A, HNF4A, KCNJ11, PGM1, PMM2, 630500 Hypoglycemia Genetic Panel SLC16A1, UCP2 Growth and Development Combined Pituitary Hormone GLI1, HESX1, LHX3, LHX4, OTX2, POU1F1, PROKR2, PROP1, -

A Bioinformatic and Mechanistic Study Elicits The
Journal name: Drug Design, Development and Therapy Article Designation: Original Research Year: 2015 Volume: 9 Drug Design, Development and Therapy Dovepress Running head verso: He et al Running head recto: UA reverses liver fibrosis in rats open access to scientific and medical research DOI: http://dx.doi.org/10.2147/DDDT.S85426 Open Access Full Text Article ORIGINAL RESEARCH A bioinformatic and mechanistic study elicits the antifibrotic effect of ursolic acid through the attenuation of oxidative stress with the involvement of ERK, PI3K/Akt, and p38 MAPK signaling pathways in human hepatic stellate cells and rat liver Wenhua He1,* Abstract: NADPH oxidases (NOXs) are a predominant mediator of redox homeostasis in Feng Shi1,* hepatic stellate cells (HSCs), and oxidative stress plays an important role in the pathogenesis Zhi-Wei Zhou2,* of liver fibrosis. Ursolic acid (UA) is a pentacyclic triterpenoid with various pharmacological Bimin Li1 activities, but the molecular targets and underlying mechanisms for its antifibrotic effect in the Kunhe Zhang1 liver remain elusive. This study aimed to computationally predict the molecular interactome Xinhua Zhang1 and mechanistically investigate the antifibrotic effect of UA on oxidative stress, with a focus on NOX4 activity and cross-linked signaling pathways in human HSCs and rat liver. Drug–drug Canhui Ouyang1 interaction via chemical–protein interactome tool, a server that can predict drug–drug interac- Shu-Feng Zhou2 tion via chemical–protein interactome, was used to predict the molecular targets of UA, and 1 Xuan Zhu Database for Annotation, Visualization, and Integrated Discovery was employed to analyze 1Department of Gastroenterology, the the signaling pathways of the predicted targets of UA.