Uncommon Forms of Diabetes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 7: Monogenic Forms of Diabetes
CHAPTER 7 MONOGENIC FORMS OF DIABETES Mark A. Sperling, MD, and Abhimanyu Garg, MD Dr. Mark A. Sperling is Emeritus Professor and Chair, University of Pittsburgh, Department of Pediatrics, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA. Dr. Abhimanyu Garg is Professor of Internal Medicine and Chief of the Division of Nutrition and Metabolic Diseases at University of Texas Southwestern Medical Center, Dallas, TX. SUMMARY Types 1 and 2 diabetes have multiple and complex genetic influences that interact with environmental triggers, such as viral infections or nutritional excesses, to result in their respective phenotypes: young, lean, and insulin-dependence for type 1 diabetes patients or older, overweight, and often manageable by lifestyle interventions and oral medications for type 2 diabetes patients. A small subset of patients, comprising ~2%–3% of all those diagnosed with diabetes, may have characteristics of either type 1 or type 2 diabetes but have single gene defects that interfere with insulin production, secretion, or action, resulting in clinical diabetes. These types of diabetes are known as MODY, originally defined as maturity-onset diabetes of youth, and severe early-onset forms, such as neonatal diabetes mellitus (NDM). Defects in genes involved in adipocyte development, differentiation, and death pathways cause lipodystrophy syndromes, which are also associated with insulin resistance and diabetes. Although these syndromes are considered rare, more awareness of these disorders and increased availability of genetic testing in clinical and research laboratories, as well as growing use of next generation, whole genome, or exome sequencing for clinically challenging phenotypes, are resulting in increased recognition. A correct diagnosis of MODY, NDM, or lipodystrophy syndromes has profound implications for treatment, genetic counseling, and prognosis. -

DIAGMOL Town, ZIPCODE :…………………………………………………… Director : Prof
Mr. Mrs. (IN UPPER CASE, please) Name:……………………………………………………. Maiden Name:……………………………………. DMGL / Service de Médecine Génétique Centre d’accueil des prélèvements (CAP) First name :………………………………………………… Bâtiment des Laboratoires (BATLab), local 8D-0-850.1 Date of birth : ............ / ........... / …………… 4 rue Gabrielle-Perret-Gentil, 1211 Genève 14 Legal representative (for minors) : father mother Molecular and Genomic Diagnostics Laboratory Name/first name :…………………………………………… Street/N°:……………………………………………………… http://www.hug-ge.ch/feuilles-de-demande DIAGMOL Town, ZIPCODE :…………………………………………………… Director : Prof. Stylianos ANTONARAKIS Hospitalisation Unit: …………… Physician :…………………… Lab managers: N° EdS : …………………………………………………………… Dr J.-L. BLOUIN, Dr Th. NOUSPIKEL, Dr. M. GUIPPONI Invoice address: Patient Prescriptor Insurance [email protected], [email protected], [email protected] Type of case : Disease AI Accident Pregnancy Lab direct or results: Phone/FAX: +41 (0) 22 37 21 826 / 21 860 N° AVS (Mandatory for AI) : ………………………...................... Sample Entrance Center (CAP) : Phone +41 (0) 22 37 21 800 Insurance : ………………… Insured N° : ……………………… PHYSICIAN PHYSICIAN (NAME/First name - Street/N°- Town, ZIPCODE - Phone/FAX. IN UPPER CASES, PLEASE) COPY TO OTHER PHYSICIAN (NAME/First name - Street/N°- Town, ZIPCODE - Phone/FAX. IN UPPER CASES, PLEASE) « The laboratory is granted permission by the Physician/Patient to transmit copies of the report to other physicians» Opposition of the patient to the registration of this request results in the electronic patient record (DPI) of the HUG If the patient belongs to a family already known to the laboratory, please indicate index case NAME: CLINICAL INFORMATIONS given by the physician: Ethnic origins Father Mother Currently pregnant Date of last menses Number of weeks of amenorrhea SAMPLE(S) Most of our tests work from 4 ml of blood in EDTA (children <2 ans : 1 On every and single NAME First name ml : ok) or from purified DNA (some exceptions apply for some tests) tube. -

Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up of Diabetes Mellitus and Its Complications - 2019
THE SOCIETY of ENDOCRINOLOGY and METABOLISM of TURKEY (SEMT) Clinical Practice Guideline for Diagnosis, Treatment and Follow-up of Diabetes Mellitus and Its Complications - 2019 English Version of the 12th Edition SEMT Diabetes Mellitus Working Group ISBN: 978-605-4011-39-1 CLINICAL PRACTICE GUIDELINE FOR DIAGNOSIS, TREATMENT, AND FOLLOW-UP OF DIABETES MELLITUS AND ITS COMPLICATIONS-2019 © SEMT -2019 This material has been published and distributed by The Society of Endocrinology and Metabolism of Turkey (SEMT). Whole or part of this guideline cannot be reproduced or used for commercial purposes without permission. THE SOCIETY of ENDOCRINOLOGY and METABOLISM of TURKEY (SEMT) Meşrutiyet Cad., Ali Bey Apt. 29/12, Kızılay 06420, Ankara, Turkey Phone. +90 312 425 2072 http://www.temd.org.tr ISBN: 978-605-4011-39-1 English Version of the 12th Edition Publishing Services BAYT Bilimsel Araştırmalar Basın Yayın ve Tanıtım Ltd. Şti. Ziya Gökalp Cad. 30/31 Kızılay 06420, Ankara, Turkey Phone. +90 312 431 3062 Fax +90 312 431 3602 www.bayt.com.tr Printing Miki Matbaacılık San. ve Tic. Ltd. Şti. Matbaacılar Sanayi Sitesi 1516/1 Sk. No. 27 İvedik, Yenimahalle / Ankara, Turkey Phone. +90 312 395 2128 Print Date: October 23, 2019 “Major achievements and important undertakings are only possible through collaborations” “Büyük işler, mühim teşebbüsler; ancak, müşterek mesai ile kabil-i temindir.” MUSTAFA KEMAL ATATÜRK, 1925 PREFACE Dear colleagues, The prevalence of diabetes has been increasing tremendously in the last few decades. As a result , medical professionals/ specialists from different fields encounter many diabetics in their daily practice. At this point, updated guidelines on diabetes management, which take regional specification into consideration is needed. -

UNSCEAR 2001 Report to the General Assembly, with Scientific Annex
HEREDITARY EFFECTS OF RADIATION United Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR 2001 Report to the General Assembly, with Scientific Annex UNITED NATIONS HEREDITARY EFFECTS OF RADIATION United Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR 2001 Report to the General Assembly, with Scientific Annex UNITED NATIONS New York, 2001 NOTE The report of the Committee without its scientific annex appears as Official Records of the General Assembly. Fifty-sixth Session, Supplement No. 46 (N56146). The designation employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the pad of the Secretariat of the United Nationsconcerning the legal status of any country, temtory, city orarea, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The country names used in this document are, In most cases, those that were in use at the time the data were collected or the text prepared. In othercases, however, the names have been updated, where this was posslble and appropriate, to reflect political changes. UNITED NATIONS PUBLICATION Sales No. E.O1 .IX.2 ISBN 92-1-1 42244-2 Report of the United Nations Scientific Committee on the Effects of Atomic Radiation to the General Assembly 1. During the past few years, the United Nations 4. The Committee wishes to acknowledge the assistance Scientific Committee on the Effects of Atomic Radiation1 of the consultant, K. Sankaranarayanan, in the preparation has undertaken broad reviews of the sources and effects of of the scientific annex and the advice of the international ionizing radiation. -

Mat Kadi Tora Tutti O Al Ut Hit Hitta Atuh
MAT KADI TORA TUTTI USO AL20180235194A1 UT HIT HITTA ATUH ( 19) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2018 /0235194 A1 Fahrenkrug et al. (43 ) Pub . Date : Aug . 23, 2018 ( 54 ) MULTIPLEX GENE EDITING Publication Classification (51 ) Int. Ci. ( 71 ) Applicant: Recombinetics , Inc ., Saint Paul, MN A01K 67/ 027 (2006 . 01 ) (US ) C12N 15 / 90 ( 2006 .01 ) (72 ) Inventors : Scott C . Fahrenkrug, Minneapolis , (52 ) U . S . CI. MN (US ) ; Daniel F . Carlson , CPC .. .. A01K 67 / 0276 (2013 . 01 ) ; C12N 15 / 907 Woodbury , MN (US ) ( 2013 .01 ) ; A01K 67 /0275 ( 2013 .01 ) ; A01K 2267/ 02 (2013 .01 ) ; AOIK 2217 / 15 (2013 .01 ) ; AOIK 2227 / 108 ( 2013 .01 ) ; AOIK 2217 /07 (21 ) Appl. No. : 15 /923 , 951 ( 2013 .01 ) ; A01K 2227/ 101 (2013 .01 ) ; AOIK ( 22 ) Filed : Mar. 16 , 2018 2217 /075 ( 2013 .01 ) (57 ) ABSTRACT Related U . S . Application Data Materials and methods for making multiplex gene edits in (62 ) Division of application No . 14 /698 ,561 , filed on Apr. cells and are presented . Further methods include animals 28 , 2015, now abandoned . and methods of making the same . Methods of making ( 60 ) Provisional application No . 61/ 985, 327, filed on Apr. chimeric animals are presented , as well as chimeric animals . 28 , 2014 . Specification includes a Sequence Listing . Patent Application Publication Aug . 23 , 2018 Sheet 1 of 13 US 2018 / 0235194 A1 GENERATION OF HOMOZYGOUS CATTLE EDITED AT ONE ALLELE USING SINGLE EDITS Edit allele , Raise FO to Mate FO enough times Raise F1s to Mate F1 siblings Clone cell , sexual maturity , to produce enough F1 sexualmaturity , to make Implant, Gestate 2 years generation carrying 2 years homozygous KO , 9 months , birth edited allele to mate 9 months of FO with each other Generation Primary Fibroblasts ? ? ? Time, years FIG . -
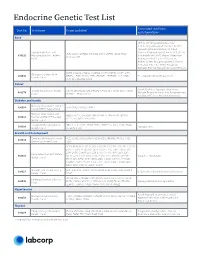
Endocrine Genetic Test List
Endocrine Genetic Test List Associated conditions Test No. Test Name Genes Included and phenotypes Bone HPP, X-Linked Hypophosphatemia, X-Linked Hypophosphatemic Rickets, XLH, Hypophosphatemic Rickets, X-Linked Hypophosphatasia and Dominant Hypophosphatemic Rickets (XLHR), ALPL, CLCN5, CYP2R1, CYP27B1, DMP1, ENPP1, FGF23, PHEX, 630292 Hypophosphatemic Rickets X-Linked Rickets (XLR), Vitamin D-Resistant SLC34A3, VDR Panel Rickets, X-Linked Vitamin D-Resistant Rickets (VDRR), Hypophosphatemic Vitamin D-Resistant Rickets (HPDR), Phosphate Diabetes, Familial Hypophosphatemic Rickets BMP1, COL1A1, COL1A2, CREB3L1, CRTAP, FKBP10, IFITM5, LRP5, Osteogenesis Imperfecta 630543 MBTPS2, P3H1, PLOD2, PPIB, SERPINF1, SERPINH1, SP7, SPARC, OI, Juvenile Primary Osteoporosis Genetic Panel TENT5A, TMEM38B, WNT1 Cancer Familial Isolated Hyperparathyroidism, VistaSeq® Endocrine Cancer CDC73, MAX, MEN1, NF1, PRKAR1A, PTEN, RET, SDHB, SDHC, SDHD, 481374 Multiple Endocrine Neoplasia, Paraganglioma, Panel* TMEM127, TP53 and VHL Parathyroid Cancer, Pheochromocytoma Diabetes and Insulin Maturity-Onset Diabetes of the 630568 GCK, HNF1A, HNF1B, HNF4A Young (MODY) 4-gene Panel Maturity-Onset Diabetes of ABCC8, APPL1, BLK, GCK, HNF1A, HNF1B, HNF4A, INS, KCNJ11, 630513 the Young (MODY) Expanded KLF11, NEUROD1, PAX4, PDX1 Genetic Panel Congenital Hyperinsulinism ABCC8, GCK, GLUD1, HADH, HNF1A, HNF4A, KCNJ11, PGM1, PMM2, 630500 Hypoglycemia Genetic Panel SLC16A1, UCP2 Growth and Development Combined Pituitary Hormone GLI1, HESX1, LHX3, LHX4, OTX2, POU1F1, PROKR2, PROP1, -

An Interesting Case of Young Onset Diabetes Mellitus
International Journal of Research in Medical Sciences Gulati S et al. Int J Res Med Sci. 2017 Sep;5(9):4178-4180 www.msjonline.org pISSN 2320-6071 | eISSN 2320-6012 DOI: http://dx.doi.org/10.18203/2320-6012.ijrms20174007 Case Report An interesting case of young onset diabetes mellitus Shipra Gulati*, Tanvi Batra, Akshay A. Dhamne, Vijayashree S. Gokhale Department of Medicine, Dr. DY Patil Medical College and Hospital, Pimpri, Pune, Maharashtra, India Received: 23 June 2017 Accepted: 22 July 2017 *Correspondence: Dr. Shipra Gulati, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT A 24 years old female, was admitted with symptoms of urinary tract infection. She was married and had bad obstetric history. She was known diabetic for 16 years of age and was on regular treatment with injection human insulin mixtard since the time of diagnosis, but had no episode of diabetic ketosis/ ketoacidosis. She had a positive family history of diabetes. She was further evaluated and was found to have normal C peptide levels and islet cell antibodies were found to be negative. Hence, the possibility of MODY (monogenic diabetes) was considered. Her genetic testing could not be done due to financial constraints. But a trial of sulfonylureas was given along with reduction in the dose of insulin to which she responded well and is presently well controlled. -

Servicios GENYCA 20171218 ENGLISH
RARE DISEASES GENYCA_2018 TAT* CODE TEST OMIM ANALYSIS OF: GENES SAMPLE SPECIALITY (turnaraound time) 5 ml blood EDTA/Saliva SRD5A1-S Steroid 5-alpha-reductase *184753 cds SRD5A1 1 month Oncologic on swab 5 ml blood EDTA/Saliva ABL1-S ABL1, Gene *189980 cds ABL1 3 months Oncologic on swab 5 ml blood EDTA/Saliva GCDH-S Glutaric Acidemia Type I 231670 cds GCDH 4 weeks Metabolic on swab ACAT1, BCKDHA, BCKDHB, DBT, 251000, 251100, 251110, GCDH, HMGCL, IVD, MMAA, ACID-N Isolated Methylmalonic Acidemia 251120, 277410, 607481, cds 5 ml blood EDTA 3 months Metabolic MMAB, MMACHC, MCCC1, 607568, 608419, 609058 MCCC2, MUT, PCCA, PCCB 5 ml blood EDTA/Saliva SLC4A1-S Renal Tubular Distal Acidosis 611590 cds SLC4A1 6 weeks Hematologic on swab 5 ml blood EDTA/Saliva AUH-S 3-methylglutaconic aciduria, type I 250950 cds AUH 2 months Metabolic on swab 5 ml blood EDTA/Saliva IDH2-S D-2-hydroxyglutaric aciduria 2 613657 cds IDH2 6 weeks Multisystemic on swab ACYL-COA Dehydrogenase Medium-Chain, c.985A>G 5 ml blood EDTA/Saliva ACADM-V 201450 ACADM 1 month Metabolic Deficiency of (p.Lys304Glu) on swab ACYL-COA Dehydrogenase Medium-Chain, ACADM-S 201450 cds ACADM 5 ml blood EDTA 2 months Metabolic Deficiency of 5 ml blood EDTA/Saliva FGFR3-V Achondroplasia 100800 p.G380R, p.G375C FGFR3 2 weeks Musculoskeletal on swab 5 ml blood EDTA/Saliva ITGB2-S Leukocyte adhesion deficiency (LAD) 116920 cds ITGB2 2 months Hematologic on swab 5 ml blood EDTA/Saliva ADIPOQ-V Adiponectin (Hipoadiponectinemia) 612556 c.276G>T ADIPOQ 1 week Metabolic on swab ABCD1-D Adrenoleukodystrophy -

Research Article DIABETIC NEPHROPATHY – a MAJOR
Available online www.ijpras.com International Journal of Pharmaceutical Research & Allied Sciences, 2016, 5(4):132-158 ISSN : 2277-3657 Research Article CODEN(USA) : IJPRPM DIABETIC NEPHROPATHY – A MAJOR MACROVASCULAR COMPLICATION Kehkashan Parveen, Waseem A. Siddiqui*, Mohd Adnan Kausara, Mohammed Kuddusa, Syed Monowar Alam Shahida, Jamal Mohammad Arifa*@ Department of Biochemistry, Lipid Metabolism Laboratory, Jamia Hamdard (Hamdard University), New Delhi–110062, INDIA aDepartment of Biochemistry, College of Medicine, University of Hail, Hail, KSA *Corresponding authors @Present Address: Prof. (Dr.) Jamal Mohammad Arif, Dean, Research and Development, Integral University, Lucknow 226 026, (U.P.) INDIA E-mail:. [email protected] ____________________________________________________________________________________________ ABSTRACT Diabetes mellitus (DM) is a complex, progressive disease, which is accompanied by multiple complications. One of the major complication confronted by patients with diabetes is an increased risk of developing diabetic nephropathy (DN) that often progresses to end-stage renal disease.Pathogenesis of DN is multifactorial. The role of hyperglycemia in the pathogenesis of DN has been previously established by a number of studies.Hyperglycemia induces oxidative stress in the rat kidney and increased oxidative stress in the kidney may trigger apoptosis in renal cells in vitro by inducing DNA fragmentation and stimulating expression of apoptosis-regulatory genes. Hyperglycemia also leads to accumulation of advanced glycation end products (AGE's) in renal cortex. These AGE's play a role in the progression of DN through impairment of matrix proteins in vivo, leading to thickening of glomerular basement membrane and expansion of mesangial matrix. DN is also associated with dyslipidemia, which is characterized by higher plasma levels of total cholesterol, low-density lipoprotein and triglycerides, and lower levels of high-density lipoprotein. -

Retinal Pigment Epithelial Change and Partial Lipodystrophy
Postgrad Med J: first published as 10.1136/pgmj.64.757.871 on 1 November 1988. Downloaded from Postgraduate Medical Journal (1988) 64, 871-874 Clinical Reports Retinal pigment epithelial change and partial lipodystrophy T.M.E. Davis,* D.R. Holdright, W.E. Schulenberg,l R.C. Turner2 and G.F. Joplin Department of Medicine and 'Department of Surgery, Royal Postgraduate Medical School, Hammersmith Hospital, Du Cane Road, London W12 OHS, and 2Diabetes Research Laboratories, Radcliffe Infirmary, Woodstock Road, Oxford OX2 6HE, UK. Summary: Cuticular drusen and retinal pigment epithelial changes were found incidentally in a 27 year old Lebanese woman during assessment of partial lipodystrophy. Her vision was normal despite involvement of both maculae. The patient had hypocomplementaemia, but serum C3 nephritic factor was absent and renal function was normal. She had impaired glucose tolerance and a continuous infusion of glucose with model assessment (CIGMA) test revealed low normal tissue insulin sensitivity and high normal pancreatic beta cell function. Mild fasting hypertriglyceridaemia (2.0 mmol/l) may have been secondary to impaired insulin sensitivity. Endocrine function was otherwise normal apart from a completely absent growth hormone response to adequate hypo- glycaemia. The simultaneous occurrence of partial lipodystrophy and retinal pigmentary epithelial and basement membrane changes appears to be a newly recognized syndrome. Introduction Case report by copyright. Partial lipodystrophy, first described over 100 years A 27 year old single Lebanese woman was referred ago' is one of several poorly-understood disorders for assessment of partial lipodystrophy. After a 3 characterized by loss of subcutaneous fat.2'3 In its year period of unexplained, progressive and most common form, partial lipodystrophy affects generalized weight loss from the age of 7 years, she the face, trunk and upper limbs in a 'cephalo- began to notice that subsequent weight gain was thoracic' distribution. -

Abstracts of Lectures Gfh ÖGH SGMG Tagungsband Abstracts
patients were significantly different across pop mosomal level, but not generally at both levels. Abstracts of Lectures ulations with frequency maximum of the com In this view, the aneuploid karyotype is the read mon mutations in EastEurope (W151X, V326L), out of an underlying chromosomal instability NorthWestEurope (IVS81G>C), and SouthEu (CIN). In a small proportion of cancers display 1. Symposia rope (T93M). ing CIN the loss of this checkpoint is associated Carrier frequency analysis of the IVS81G>C, with the mutational inactivation of a human ho W151X, T93M, and V326L mutations in 2250 mologue of the yeast BUB1 gene. BUB1 controls S1 healthy individuals from different European pop mitotic checkpoints and chromosome segrega ulations revealed much higher frequencies for tion in yeast. The Human SHOX Mutation Database these common mutations (e.g. 1:50 for the IVS8 Because the MIN and CIN forms of instability are Beate Niesler, Christine Fischer and Gudrun A. 1G>C in Austria, and 1:84 for the W151X in rarely found to coexist in tumours, it would seem Rappold Poland) than expected from the reported preva that one form of instability is sufficient to drive Institute of Human Genetics, University of lence of the SLOS. Based on these frequencies tumorigenesis. Heidelberg, Im Neuenheimer Feld 328, the expected incidence of SLOS patients with Genetic instability appears early in tumorigene 69120 Heidelberg, Germany null mutations ranges from 1:2000 to 1:16.000. sis and is believed to play a critical role in the The human SHOX gene (Short Stature Home This discrepancy might be due to underdiagno malignant process. -

Lipoatrophic Diabetes in Irs1 /Irs3 Double Knockout Mice
Downloaded from genesdev.cshlp.org on September 25, 2021 - Published by Cold Spring Harbor Laboratory Press Lipoatrophic diabetes in Irs1−/−/Irs3−/− double knockout mice Palle G. Laustsen,1,3 M. Dodson Michael,1,3 Barbara E. Crute,2 Shmuel E. Cohen,1 Kohjiro Ueki,1 Rohit N. Kulkarni,1 Susanna R. Keller,2 Gustav E. Lienhard,2 and C. Ronald Kahn1,4 1Joslin Diabetes Center and Department of Medicine, Harvard Medical School, Boston, Massachusetts 02215, USA; 2Department of Biochemistry, Dartmouth Medical School, Hanover, New Hampshire 03755, USA Based on the phenotypes of knockout mice and cell lines, as well as pathway-specific analysis, the insulin receptor substrates IRS-1, IRS-2, IRS-3, and IRS-4 have been shown to play unique roles in insulin signal transduction. To investigate possible functional complementarity within the IRS family, we generated mice with double knockout of the genes for IRS-1/IRS-3 and IRS-1/IRS-4. Mice with a combined deficiency of IRS-1 and IRS-4 showed no differences from Irs1−/− mice with respect to growth and glucose homeostasis. In contrast, mice with a combined deficiency of IRS-1 and IRS-3 developed early-onset severe lipoatrophy associated with marked hyperglycemia, hyperinsulinemia, and insulin resistance. However, in contrast to other models of lipoatrophic diabetes, there was no accumulation of fat in liver or muscle. Furthermore, plasma leptin levels were markedly decreased, and adenovirus-mediated expression of leptin in liver reversed the hyperglycemia and hyperinsulinemia. The results indicate that IRS-1 and IRS-3 play important complementary roles in adipogenesis and establish the Irs1−/−/Irs3−/− double knockout mouse as a novel model of lipoatrophic diabetes.