Chapter 4 Artificial Radioactive Isotopes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Synchrotrons in Britain, and Early Work for Cern
EARLY SYNCHROTRONS IN BRITAIN, AND EARLY WORK FOR CERN J. D. Lawson Formerly Rutherford Appleton Laboratory, Chilton, Oxon, UK Abstract Early work on electron synchrotrons in the UK, including an account of the conversion of a small betatron in 1946 to become the world’s first synchrotron, is described first. This is followed by a description of the design and construction of the 1 GeV synchrotron at the University of Birmingham which was started in the same year. Finally an account is given of the work of the international team during 1952–3, which formed the basis for the design of the CERN PS before the move to Geneva. It was during this year that John Adams showed the outstanding ability that later brought the project to such a successful conclusion. 1 EARLY PLANS IN BRITAIN: THE WORLD’S FIRST SYNCHROTRON During the second world war Britain’s nuclear physicists were deployed in research directed towards winning the war. Many were engaged in developments associated with radar, (or ‘radiolocation’ as it was then called), both at universities and at government laboratories, such as the radar establishments TRE and ADRDE at Malvern. Others contributed to the atomic bomb programme, both in the UK, and in the USA. Towards the end of the war, when victory seemed assured, the nuclear physicists began looking towards the peacetime future. The construction of new particle accelerators to achieve ever higher energies was seen as one of the more important possibilities. Those working at Berkeley on the electromagnetic separator were familiar with the accelerators there, and following the independent invention (or discovery?) there of the principle of phase stability by Edwin McMillan in 1945, exciting possibilities were immediately apparent [4]. -

MASTER NATIONAL ACADEMY PRESS Washington, D.C 1983
OPPORTUNITIES AND CHALLENGES IN C0NP 830214 RESEARCH WITH TRANSPLUTONIUM ELEMENTS DE85 010852 Board on Chemical Sciences and Technology Committee on Nuclear and Radlochemistry Commission on Physical Sciences, Mathematics, and Resources National Research Council DISCLAIMER This iw.oort was prepared as an account of work spcnsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsi- bility for the accuracy, completeness, or usefulness of any infonnation, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Refer- ence herein to any specific commercial product, process, or scivice by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recom- mendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. MASTER NATIONAL ACADEMY PRESS Washington, D.C 1983 DISTBIBUHOU OF THIS DOCUMENT IS Workshop Steering Committee Gerhart Friedlander, Brookhaven National Laboratory, Chairman Gregory R. Choppin, Florida State University Richard L. Hoff, Lawrence Livermore National Laboratory Darleane C. Hoffman, Los Alamos Scientific Laboratory, Ex-Officio James A. Ibers, Northwestern University Robert A. Penneman, Los Alamos Scientific Laboratory Thomas G. Spiro, Princeton University Henry Taube, Stanford University Joseph Weneser, Brookhaven National Laboratory Raymond G. Wymer, Oak Ridge National Laboratory, Ex-Officio NRC Staff William Spindel, Executive Secretary Peggy J. Posey, Staff Associate Robert M. -

Otto Hahn Otto Hahn
R.N. 70269/98 Postal Registration No.: DL-SW-1/4082/15-17 ISSN : 0972-169X Date of posting: 26-27 of advance month Date of publication: 24 of advance month May 2017 Vol. 19 No. 8 Rs. 5.00 Otto Hahn Discoverer of Nuclear Fission Editorial: Consolidating 35 science communication activities in our country Otto Hahn: Discoverer of 34 Nuclear Fission Keep Your Eyes Healthy 31 Phenol: A Serious 30 Environmental Threat Accidental Discoveries in 28 Medical Science Cures for haemorrhoids— 24 Simple treatments and Surgeries Recent developments 21 in science and technology 36 Editorial Consolidating science communication activities in our country Dr. R. Gopichandran It is well known that the National Council of Science Museums of India’s leadership in science technology and innovation (STI) across the Ministry of Culture, Government of India, the National Institute the bilateral and multilateral framework also. The news feature service of Science Communication and Information Resources (NISCAIR) and the portal activity have well defined action plans to reach out to of CSIR, the National Council for Science and Technology fellow institutions and citizens with suitably embellished platform Communication (NCSTC) of the Department of Science and and opportunities for all to deliver together. Technology (DST), Government of India and Vigyan Prasar, also While these are interesting and extremely important, especially of DST, have been carrying out excellent science communication because they respond to the call to upscale and value add science activities over the years. It cannot be denied that the reach has been and technology communication, it is equally important to document quite significant collectively. -

Appendix E Nobel Prizes in Nuclear Science
Nuclear Science—A Guide to the Nuclear Science Wall Chart ©2018 Contemporary Physics Education Project (CPEP) Appendix E Nobel Prizes in Nuclear Science Many Nobel Prizes have been awarded for nuclear research and instrumentation. The field has spun off: particle physics, nuclear astrophysics, nuclear power reactors, nuclear medicine, and nuclear weapons. Understanding how the nucleus works and applying that knowledge to technology has been one of the most significant accomplishments of twentieth century scientific research. Each prize was awarded for physics unless otherwise noted. Name(s) Discovery Year Henri Becquerel, Pierre Discovered spontaneous radioactivity 1903 Curie, and Marie Curie Ernest Rutherford Work on the disintegration of the elements and 1908 chemistry of radioactive elements (chem) Marie Curie Discovery of radium and polonium 1911 (chem) Frederick Soddy Work on chemistry of radioactive substances 1921 including the origin and nature of radioactive (chem) isotopes Francis Aston Discovery of isotopes in many non-radioactive 1922 elements, also enunciated the whole-number rule of (chem) atomic masses Charles Wilson Development of the cloud chamber for detecting 1927 charged particles Harold Urey Discovery of heavy hydrogen (deuterium) 1934 (chem) Frederic Joliot and Synthesis of several new radioactive elements 1935 Irene Joliot-Curie (chem) James Chadwick Discovery of the neutron 1935 Carl David Anderson Discovery of the positron 1936 Enrico Fermi New radioactive elements produced by neutron 1938 irradiation Ernest Lawrence -

Regional Oral History Office University of California the Bancroft Library Berkeley, California
Regional Oral History Office University of California The Bancroft Library Berkeley, California Program in Bioscience and Biotechnology Studies RONALD E. CAPE, M.B.A., Ph. D. BIOTECH PIONEER AND CO-FOUNDER OF CETUS Interviews Conducted by Sally Smith Hughes in 2003 Copyright © 2006 by The Regents of the University of California Since 1954 the Regional Oral History Office has been interviewing leading participants in or well-placed witnesses to major events in the development of northern California, the West, and the nation. Oral history is a method of collecting historical information through tape-recorded interviews between a narrator with firsthand knowledge of historically significant events and a well-informed interviewer, with the goal of preserving substantive additions to the historical record. The tape recording is transcribed, lightly edited for continuity and clarity, and reviewed by the interviewee. The corrected manuscript is indexed, bound with photographs and illustrative materials, and placed in The Bancroft Library at the University of California, Berkeley, and in other research collections for scholarly use. Because it is primary material, oral history is not intended to present the final, verified, or complete narrative of events. It is a spoken account, offered by the interviewee in response to questioning, and as such it is reflective, partisan, deeply involved, and irreplaceable. ************************************ All uses of this manuscript are covered by legal agreements between The Regents of the University of California and Ronald Cape, dated December 18, 2003. The manuscript is thereby made available for research purposes. All literary rights in the manuscript, including the right to publish, are reserved to The Bancroft Library of the University of California, Berkeley. -

Recollections and Reconstructions
@ ? Editor's Note: The Society's Nuclear Pioneer Citationfor 1977 goes not to an individual but to a distinguished group of individuals: those who took part in the epochal “ChicagoPile―experiment of Dec. 2, 1942, which produced thefirst successful atomicpile chain reaction. The 42 members ofthe Chicago Pilegroup are listed on page 591. One ofthose named, Harold M. Agnew, now Directorofthe Los Alamos Scient@flc Laboratory, is the Nuclear Pioneer Lecturerfor the 24th Annual Meeting. The guidingforce behind the Chicago Pile experiment was Enrico Fermi, recipient of the Nuclear Pioneer Citation of 1963. In the artick which follows, Laura Fermi recreates the excitement and mystery that surrounded the work ofher husband's research team in the early 1940s in Chicago. @. The FirstAtomic Pile: Recollections and Reconstructions by Laura Fermi @ . @ / - .-i@ ‘@:@ Enrico FermIat work, ca. 1942. As far as I can remember at the distance ofalmost 35 Perhaps not all husbands adhered as strictly as years, the first guests to arrive at our party on that Enrico to the rules of secrecy when talking to their beastly cold December night were the Zinns. Wally wives. He couldn't have been more tight-lipped. Once! and Jean. As they shook the snow off their coats, related some gossip to him: “Peoplesay that you stamping their feet on the floor, Wally turned to scientists at the Met Lab are seeking a cure for Enrico and said: “Congratulations!― I was surprised; cancer. .““Arewe?―he asked with his usual for Enrico had given me no hint that anything unusual imperturbable expression. -

MIT-NSE by the 1930S It Was Time to Discover Fission. We Knew About Isoto
Prelude to the Manhattan Project Michael V. Hynes – MIT-NSE By the 1930s it was time to discover fission. We knew about isotopes. The Curies and Becquerel had discovered radioactivity. Rutherford had discovered the nucleus. Chadwick had discovered the neutron. Einstein had discovered special relativity and the famous equation E=mc2. Bethe was even publishing about fusion as the source of energy for the Sun. So, it was time. By the 1930s a human drama was unfolding in Europe and in the Pacific – the rise of fascism. The events of this drama engulfed the world in war and created a need on all sides for weaponry that could defeat the enemy by whatever means. The intersection of scientific inevitability and war created the environment for the advent of nuclear weapons. Science was practiced was very differently in the 1920s and 1930s than today. In that era science was highly specialized and compartmentalized. If you were a metallurgist for example it was unlikely that you would have anything to do with a physicist – that all changed in the1940s. In 1933 Leo Szilard (Fig. 1), a Hungarian physicist who took refuge in London from Nazi Germany, read a paper by Rutherford that ridiculed the idea of getting energy from nuclear transmutations. Szilard realized that if you could find an element which is split by neutrons and which would emit two neutrons in the process, then a chain reaction could be started. This is the basic idea of a nuclear weapon -- to generate energy from the chain reaction. Szilard was a chemist by training and knew about the idea of a chemical chain reaction. -
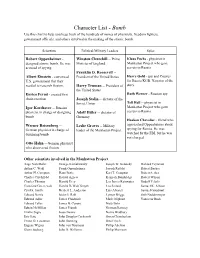
Character List
Character List - Bomb Use this chart to help you keep track of the hundreds of names of physicists, freedom fighters, government officials, and others involved in the making of the atomic bomb. Scientists Political/Military Leaders Spies Robert Oppenheimer - Winston Churchill -- Prime Klaus Fuchs - physicist in designed atomic bomb. He was Minister of England Manhattan Project who gave accused of spying. secrets to Russia Franklin D. Roosevelt -- Albert Einstein - convinced President of the United States Harry Gold - spy and Courier U.S. government that they for Russia KGB. Narrator of the needed to research fission. Harry Truman -- President of story the United States Enrico Fermi - created first Ruth Werner - Russian spy chain reaction Joseph Stalin -- dictator of the Tell Hall -- physicist in Soviet Union Igor Korchatov -- Russian Manhattan Project who gave physicist in charge of designing Adolf Hitler -- dictator of secrets to Russia bomb Germany Haakon Chevalier - friend who Werner Reisenberg -- Leslie Groves -- Military approached Oppenheimer about German physicist in charge of leader of the Manhattan Project spying for Russia. He was designing bomb watched by the FBI, but he was not charged. Otto Hahn -- German physicist who discovered fission Other scientists involved in the Manhattan Project: Aage Niels Bohr George Kistiakowsky Joseph W. Kennedy Richard Feynman Arthur C. Wahl Frank Oppenheimer Joseph Rotblat Robert Bacher Arthur H. Compton Hans Bethe Karl T. Compton Robert Serber Charles Critchfield Harold Agnew Kenneth Bainbridge Robert Wilson Charles Thomas Harold Urey Leo James Rainwater Rudolf Pelerls Crawford Greenewalt Harold DeWolf Smyth Leo Szilard Samuel K. Allison Cyril S. Smith Herbert L. Anderson Luis Alvarez Samuel Goudsmit Edward Norris Isidor I. -

Hitler's Uranium Club, the Secret Recordings at Farm Hall
HITLER’S URANIUM CLUB DER FARMHALLER NOBELPREIS-SONG (Melodie: Studio of seiner Reis) Detained since more than half a year Ein jeder weiss, das Unglueck kam Sind Hahn und wir in Farm Hall hier. Infolge splitting von Uran, Und fragt man wer is Schuld daran Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. The real reason nebenbei Die energy macht alles waermer. Ist weil we worked on nuclei. Only die Schweden werden aermer. Und fragt man, wer ist Schuld daran, Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. Die nuclei waren fuer den Krieg Auf akademisches Geheiss Und fuer den allgemeinen Sieg. Kriegt Deutschland einen Nobel-Preis. Und fragt man, wer ist Schuld daran, Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. Wie ist das moeglich, fragt man sich, In Oxford Street, da lebt ein Wesen, The story seems wunderlich. Die wird das heut’ mit Thraenen lesen. Und fragt man, wer ist Schuld daran Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. Die Feldherrn, Staatschefs, Zeitungsknaben, Es fehlte damals nur ein atom, Ihn everyday im Munde haben. Haett er gesagt: I marry you madam. Und fragt man, wer ist Schuld daran, Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. Even the sweethearts in the world(s) Dies ist nur unsre-erste Feier, Sie nennen sich jetzt: “Atom-girls.” Ich glaub die Sache wird noch teuer, Und fragt man, wer ist Schuld daran, Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. -

Edwin M. Mcmillan, a Biographical Sketch
LBL 35965 HQDFAN668 EDWIN M. MCMILLAN, A BIOGRAPHICAL SKETCH EDWARD J. LOFGREN, Associate Director Emeritus LAWRENCE BERKELEY LABORATORY BERKELEY, CALIFORNIA 94720 Presented at the International Symposium: The 50th Anniversary of the Phase Stability Principle Moscow/Dubna, Russia 12-15 July 1994 ABSTRACT. Edwin M. McMillan was one of the great scientists of the middle years of this century. He made notable contributions to nuclear, and particle physics, the chemis try of transuranic elements, and accelerator physics. * This work supported by the Director, Office of Energy Research, Office of Fusion Energy, U.S. Department of Energy under contract No. DE-AC03-76SF00098. ^-TWBUTION OF THIS DOCUMENT (S UNLIMITED Edwin McMillan was born on Septem tion Laboratory. He joined the Laboratory in ber 18,1907 at Redondo Beach, California. Soon 1934 and began a lifelong close association with after, the family moved to Pasadena, California Lawrence. where the father practised medicine for many He quickly established himself as a me years. The move to Pasadena was a very fortu ticulous and versatile experimenter in nuclear nate one because Caltech was nearby and even physics with an excellent grasp of theory. Dur in his early school years Ed could take advan ing this period he discovered *-0 with Stanley tage of the excellent public programs and lec Livingston and 10Be with Samuel Ruben. Per tures put on there by them. This contact with the haps his best experiment at that time was the world of science as Ed grew from boyhood to a demonstration of electron pair production by the young man was very important, for it nurtured absorption of gamma rays from fluorine bom the wide and lasting curiosity and interest that barded by protons from the cyclotron. -
Nobel Laureate Glenn T. Seaborg Honored with Endowed Chair; Chemistry Professor Alexander Pines Named Chairholder
04.20.98 - Nobel Laureate Glenn T. Seaborg honored with end... http://www.berkeley.edu/news/media/releases/98legacy/04_20_... NEWS RELEASE, 04/20/98 Nobel Laureate Glenn T. Seaborg honored with endowed chair; chemistry Professor Alexander Pines named chairholder By Jane Scheiber BERKELEY -- Nobel Laureate Glenn T. Seaborg, who last year became the first living scientist to have an element named for him, has been honored again, this time with the establishment of the Glenn T. Seaborg Chair in Physical Chemistry at the University of California, Berkeley. The Chair was created through a generous endowment in tribute to Seaborg and the College of Chemistry. Endowed chairs, which provide a source of income to advance the scholarly activities of the appointed professor, also are one of the highest forms of campus recognition that a faculty member can receive. Alexander Pines, who has significantly advanced the field of nuclear magnetic resonance (NMR) spectroscopy, has been named to hold the Seaborg Chair. "It is a privilege indeed to be thus associated with my legendary colleague and good friend Glenn Seaborg," Pines says. "It is especially fitting that Glenn Seaborg, who has received the highest awards of the international and national scientific communities, be thus recognized at the institution where he earned his doctorate and where he has spent most of his career," said College of Chemistry Dean Alexis T. Bell. "We are enormously grateful to our anonymous benefactors." Bell added, "We anticipate that we will be able to recognize other distinguished faculty members as additional chairs are established in the future." Seaborg earned his Ph.D. -

Edwin Mcmillan Spent a Large Part of His Professional Life in Close Association with Ernest O
NATIONAL ACADEMY OF SCIENCES E D W I N M A T T I S O N M CMILLAN 1907—1991 A Biographical Memoir by J . DAVID JACKSON AN D W . K .H. PANOFSKY Any opinions expressed in this memoir are those of the author(s) and do not necessarily reflect the views of the National Academy of Sciences. Biographical Memoir COPYRIGHT 1996 NATIONAL ACADEMIES PRESS WASHINGTON D.C. Courtesy of the Lawrence Berkeley Laboratory EDWIN MATTISON MCMILLAN September 18, 1907–September 8, 1991 BY J. DAVID JACKSON AND W. K. H. PANOFSKY ITH THE DEATH OF Edwin Mattison McMillan on Sep- Wtember 8, 1991, the world lost one of its great natural scientists. We advisedly use the term “natural scientist” since McMillan’s interests transcended greatly that of his profes- sion of physicist. They encompassed everything natural from rocks through elementary particles to pure mathematics and included an insatiable appetite for understanding ev- erything from fundamental principles. Edwin McMillan spent a large part of his professional life in close association with Ernest O. Lawrence1 and succeeded Lawrence as director of what is now the Lawrence Berkeley Laboratory in 1958. Yet the two men could hardly be more different. Lawrence was a man of great intuition, outgoing, and a highly capable organizer of the work of many people. Edwin McMillan was thoroughly analytical in whatever he did and usually worked alone or with few associates. He disliked specialization and the division of physics divided into theory and experiment. He remarked at an interna- tional high-energy physics meeting, “Any experimentalist, unless proven a damn fool, should be given one half year to interpret his own experiment.” McMillan’s first and last publications illustrate the un- usual breadth of his interests.