Rutherford and Radon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

James Chadwick: Ahead of His Time
July 15, 2020 James Chadwick: ahead of his time Gerhard Ecker University of Vienna, Faculty of Physics Boltzmanngasse 5, A-1090 Wien, Austria Abstract James Chadwick is known for his discovery of the neutron. Many of his earlier findings and ideas in the context of weak and strong nuclear forces are much less known. This biographical sketch attempts to highlight the achievements of a scientist who paved the way for contemporary subatomic physics. arXiv:2007.06926v1 [physics.hist-ph] 14 Jul 2020 1 Early years James Chadwick was born on Oct. 20, 1891 in Bollington, Cheshire in the northwest of England, as the eldest son of John Joseph Chadwick and his wife Anne Mary. His father was a cotton spinner while his mother worked as a domestic servant. In 1895 the parents left Bollington to seek a better life in Manchester. James was left behind in the care of his grandparents, a parallel with his famous predecessor Isaac Newton who also grew up with his grandmother. It might be an interesting topic for sociologists of science to find out whether there is a correlation between children educated by their grandmothers and future scientific geniuses. James attended Bollington Cross School. He was very attached to his grandmother, much less to his parents. Nevertheless, he joined his parents in Manchester around 1902 but found it difficult to adjust to the new environment. The family felt they could not afford to send James to Manchester Grammar School although he had been offered a scholarship. Instead, he attended the less prestigious Central Grammar School where the teaching was actually very good, as Chadwick later emphasised. -

Physics Connections
Charles Tracy L P S / y a a w s n e v a R n a v v e l t e D Physics connections Last year we celebrated the International Year of Physics — time and light. In particular, it described how space also known as Einstein Year. In the century since Einstein’s becomes distorted near a massive object such as a star, so that light follows a curved path rather than annus mirabilis (see CATALYST Vol. 16, No. 1) there has travelling in a straight line (Figure 1). been a revolution in the study of physics. This article explores Its publication in wartime and the fact that it was the links between some of the architects of this revolution. written in German meant that few people read it. However, one person who did read it was the British astronomer Arthur Eddington. The story goes that it was Eddington who brought Einstein to the world’s attention — though Einstein might have disagreed. instein did not become instantly famous in Eddington was a conscientious objector. As such, GCSE key words 1905; it took several years before he became a he should have spent the war in jail. Instead, he got Gravity Eglobal icon. In 1915, while Europe was caught official permission to prepare for a trip to observe a Alpha particle scattering up in the First World War, Einstein broadened his total eclipse of the Sun — a rare event. His intention Nuclear fission special theory of relativity. His general theory of was to verify Einstein’s general theory of relativity. -

Case Studies in Environmental Medicine
ATSDR Case Studies in Environmental Medicine Radon Toxicity CS213021-A AGENCY FOR TOXIC SUBSTANCES AND DISEASE REGISTRY CASE STUDIES IN ENVIRONMENTAL MEDICINE (CSEM) Radon Toxicity Course : CB/WB1585 CE Original Date: 06/01/2010 CE Expiration Date: 06/01/2012 Key Concepts • The U. S. Environmental Protection Agency estimates that indoor radon exposure may result in 21,000 lung cancer deaths annually in the United States. • Radon may be second only to smoking as a cause of lung cancer. Increased use of medical radiation also contributes to the annual radiation dose. • The combination of smoking and radon exposure results in a higher health risk. • Current technology can easily decrease the concentration of radon in indoor air, and radon’s associated risk for producing lung cancer. About This This educational case study document is one in a series of and Other self-instructional modules designed to increase the primary Case Studies care provider’s knowledge of hazardous substances in the in environment and to promote the adoption of medical Environmental practices that aid in the evaluation and care of potentially Medicine exposed patients. The complete series of Case Studies in Environmental Medicine is on the ATSDR Web site at: http://www.atsdr.cdc.gov/emes/topics/. In addition, the downloadable PDF version of this educational series and other environmental medicine materials provide content in an electronic, printable format, especially for those who may lack adequate Internet service. How to Apply See Internet address: http://www2.cdc.gov/atsdrce/ for for and more information about continuing medical education Receive credits, continuing nursing education credits, and other Agency for Toxic Substances and Disease Registry Radon Toxicity Case Studies in Environmental Medicine (CSEM) Continuing continuing education units. -

Otto Hahn Otto Hahn
R.N. 70269/98 Postal Registration No.: DL-SW-1/4082/15-17 ISSN : 0972-169X Date of posting: 26-27 of advance month Date of publication: 24 of advance month May 2017 Vol. 19 No. 8 Rs. 5.00 Otto Hahn Discoverer of Nuclear Fission Editorial: Consolidating 35 science communication activities in our country Otto Hahn: Discoverer of 34 Nuclear Fission Keep Your Eyes Healthy 31 Phenol: A Serious 30 Environmental Threat Accidental Discoveries in 28 Medical Science Cures for haemorrhoids— 24 Simple treatments and Surgeries Recent developments 21 in science and technology 36 Editorial Consolidating science communication activities in our country Dr. R. Gopichandran It is well known that the National Council of Science Museums of India’s leadership in science technology and innovation (STI) across the Ministry of Culture, Government of India, the National Institute the bilateral and multilateral framework also. The news feature service of Science Communication and Information Resources (NISCAIR) and the portal activity have well defined action plans to reach out to of CSIR, the National Council for Science and Technology fellow institutions and citizens with suitably embellished platform Communication (NCSTC) of the Department of Science and and opportunities for all to deliver together. Technology (DST), Government of India and Vigyan Prasar, also While these are interesting and extremely important, especially of DST, have been carrying out excellent science communication because they respond to the call to upscale and value add science activities over the years. It cannot be denied that the reach has been and technology communication, it is equally important to document quite significant collectively. -

Appendix E Nobel Prizes in Nuclear Science
Nuclear Science—A Guide to the Nuclear Science Wall Chart ©2018 Contemporary Physics Education Project (CPEP) Appendix E Nobel Prizes in Nuclear Science Many Nobel Prizes have been awarded for nuclear research and instrumentation. The field has spun off: particle physics, nuclear astrophysics, nuclear power reactors, nuclear medicine, and nuclear weapons. Understanding how the nucleus works and applying that knowledge to technology has been one of the most significant accomplishments of twentieth century scientific research. Each prize was awarded for physics unless otherwise noted. Name(s) Discovery Year Henri Becquerel, Pierre Discovered spontaneous radioactivity 1903 Curie, and Marie Curie Ernest Rutherford Work on the disintegration of the elements and 1908 chemistry of radioactive elements (chem) Marie Curie Discovery of radium and polonium 1911 (chem) Frederick Soddy Work on chemistry of radioactive substances 1921 including the origin and nature of radioactive (chem) isotopes Francis Aston Discovery of isotopes in many non-radioactive 1922 elements, also enunciated the whole-number rule of (chem) atomic masses Charles Wilson Development of the cloud chamber for detecting 1927 charged particles Harold Urey Discovery of heavy hydrogen (deuterium) 1934 (chem) Frederic Joliot and Synthesis of several new radioactive elements 1935 Irene Joliot-Curie (chem) James Chadwick Discovery of the neutron 1935 Carl David Anderson Discovery of the positron 1936 Enrico Fermi New radioactive elements produced by neutron 1938 irradiation Ernest Lawrence -

Chapter 1 the Biography of a Trafficking Material
Trafficking Materials and Maria Rentetzi Gendered Experimental Practices Chapter 1 The Biography of a Trafficking Material In 1904, an article titled "Radium and Radioactivity" appeared in Century 1 Magazine, a monthly popular magazine published from 1881 to 1930. The article presents Marie Curie's personal account of the discovery of radium and radioactivity. In the article, Curie discusses in depth her arduous attempts to study the radiation of the compounds of uranium and that of known chemical elements, hoping to discover more which are endowed with atomic radioactivity. She revels that it was the chemists who supplied her with the materials she needed. "As I desired to make a very thorough investigation, I had resource to different chemists, who put at my disposal specimens—in some cases the only ones in existence—containing very rare elements." Her next step was to examine different minerals, especially the oxide of uranium 2 ore (pitchblende). To her great surprise, this specimen was found to be four times more active than oxide of uranium itself. The explanation was more than obvious. "The ore must contain a substance more radioactive than uranium and thorium, and this substance must necessarily be a chemical element as yet unknown."1 Her attempts to isolate the new element would lead Marie and Pierre Curie, who joined her research shortly after, to the discovery of both polonium and radium, to the Nobel Prize in Physics in 1903, and to a second Nobel Prize in 1911, this time in chemistry. This chapter attempts to present a cultural -

Recollections and Reconstructions
@ ? Editor's Note: The Society's Nuclear Pioneer Citationfor 1977 goes not to an individual but to a distinguished group of individuals: those who took part in the epochal “ChicagoPile―experiment of Dec. 2, 1942, which produced thefirst successful atomicpile chain reaction. The 42 members ofthe Chicago Pilegroup are listed on page 591. One ofthose named, Harold M. Agnew, now Directorofthe Los Alamos Scient@flc Laboratory, is the Nuclear Pioneer Lecturerfor the 24th Annual Meeting. The guidingforce behind the Chicago Pile experiment was Enrico Fermi, recipient of the Nuclear Pioneer Citation of 1963. In the artick which follows, Laura Fermi recreates the excitement and mystery that surrounded the work ofher husband's research team in the early 1940s in Chicago. @. The FirstAtomic Pile: Recollections and Reconstructions by Laura Fermi @ . @ / - .-i@ ‘@:@ Enrico FermIat work, ca. 1942. As far as I can remember at the distance ofalmost 35 Perhaps not all husbands adhered as strictly as years, the first guests to arrive at our party on that Enrico to the rules of secrecy when talking to their beastly cold December night were the Zinns. Wally wives. He couldn't have been more tight-lipped. Once! and Jean. As they shook the snow off their coats, related some gossip to him: “Peoplesay that you stamping their feet on the floor, Wally turned to scientists at the Met Lab are seeking a cure for Enrico and said: “Congratulations!― I was surprised; cancer. .““Arewe?―he asked with his usual for Enrico had given me no hint that anything unusual imperturbable expression. -

MIT-NSE by the 1930S It Was Time to Discover Fission. We Knew About Isoto
Prelude to the Manhattan Project Michael V. Hynes – MIT-NSE By the 1930s it was time to discover fission. We knew about isotopes. The Curies and Becquerel had discovered radioactivity. Rutherford had discovered the nucleus. Chadwick had discovered the neutron. Einstein had discovered special relativity and the famous equation E=mc2. Bethe was even publishing about fusion as the source of energy for the Sun. So, it was time. By the 1930s a human drama was unfolding in Europe and in the Pacific – the rise of fascism. The events of this drama engulfed the world in war and created a need on all sides for weaponry that could defeat the enemy by whatever means. The intersection of scientific inevitability and war created the environment for the advent of nuclear weapons. Science was practiced was very differently in the 1920s and 1930s than today. In that era science was highly specialized and compartmentalized. If you were a metallurgist for example it was unlikely that you would have anything to do with a physicist – that all changed in the1940s. In 1933 Leo Szilard (Fig. 1), a Hungarian physicist who took refuge in London from Nazi Germany, read a paper by Rutherford that ridiculed the idea of getting energy from nuclear transmutations. Szilard realized that if you could find an element which is split by neutrons and which would emit two neutrons in the process, then a chain reaction could be started. This is the basic idea of a nuclear weapon -- to generate energy from the chain reaction. Szilard was a chemist by training and knew about the idea of a chemical chain reaction. -

(Owen Willans) Richardson
O. W. (Owen Willans) Richardson: An Inventory of His Papers at the Harry Ransom Center Descriptive Summary Creator: Richardson, O. W. (Owen Willans), 1879-1959 Title: O. W. (Owen Willans) Richardson Papers Dates: 1898-1958 (bulk 1920-1940) Extent: 112 document boxes, 2 oversize boxes (49.04 linear feet), 1 oversize folder (osf), 5 galley folders (gf) Abstract: The papers of Sir O. W. (Owen Willans) Richardson, the Nobel Prize-winning British physicist who pioneered the field of thermionics, contain research materials and drafts of his writings, correspondence, as well as letters and writings from numerous distinguished fellow scientists. Call Number: MS-3522 Language: Primarily English; some works and correspondence written in French, German, or Italian . Note: The Ransom Center gratefully acknowledges the assistance of the Center for History of Physics, American Institute of Physics, which provided funds to support the processing and cataloging of this collection. Access: Open for research Administrative Information Additional The Richardson Papers were microfilmed and are available on 76 Physical Format reels. Each item has a unique identifying number (W-xxxx, L-xxxx, Available: R-xxxx, or M-xxxx) that corresponds to the microfilm. This number was recorded on the file folders housing the papers and can also be found on catalog slips present with each item. Acquisition: Purchase, 1961 (R43, R44) and Gift, 2005 Processed by: Tessa Klink and Joan Sibley, 2014 Repository: The University of Texas at Austin, Harry Ransom Center Richardson, O. W. (Owen Willans), 1879-1959 MS-3522 2 Richardson, O. W. (Owen Willans), 1879-1959 MS-3522 Biographical Sketch The English physicist Owen Willans Richardson, who pioneered the field of thermionics, was also known for his work on photoelectricity, spectroscopy, ultraviolet and X-ray radiation, the electron theory, and quantum theory. -
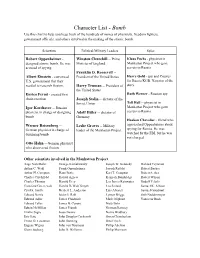
Character List
Character List - Bomb Use this chart to help you keep track of the hundreds of names of physicists, freedom fighters, government officials, and others involved in the making of the atomic bomb. Scientists Political/Military Leaders Spies Robert Oppenheimer - Winston Churchill -- Prime Klaus Fuchs - physicist in designed atomic bomb. He was Minister of England Manhattan Project who gave accused of spying. secrets to Russia Franklin D. Roosevelt -- Albert Einstein - convinced President of the United States Harry Gold - spy and Courier U.S. government that they for Russia KGB. Narrator of the needed to research fission. Harry Truman -- President of story the United States Enrico Fermi - created first Ruth Werner - Russian spy chain reaction Joseph Stalin -- dictator of the Tell Hall -- physicist in Soviet Union Igor Korchatov -- Russian Manhattan Project who gave physicist in charge of designing Adolf Hitler -- dictator of secrets to Russia bomb Germany Haakon Chevalier - friend who Werner Reisenberg -- Leslie Groves -- Military approached Oppenheimer about German physicist in charge of leader of the Manhattan Project spying for Russia. He was designing bomb watched by the FBI, but he was not charged. Otto Hahn -- German physicist who discovered fission Other scientists involved in the Manhattan Project: Aage Niels Bohr George Kistiakowsky Joseph W. Kennedy Richard Feynman Arthur C. Wahl Frank Oppenheimer Joseph Rotblat Robert Bacher Arthur H. Compton Hans Bethe Karl T. Compton Robert Serber Charles Critchfield Harold Agnew Kenneth Bainbridge Robert Wilson Charles Thomas Harold Urey Leo James Rainwater Rudolf Pelerls Crawford Greenewalt Harold DeWolf Smyth Leo Szilard Samuel K. Allison Cyril S. Smith Herbert L. Anderson Luis Alvarez Samuel Goudsmit Edward Norris Isidor I. -

Commentary & Notes Hans Bethe
J. Natn.Sci.Foundation Sri Lanka 2005 33(1): 57-58 COMMENTARY & NOTES HANS BETHE (1906-2005) Hans Albrecht Bethe who died on 6 March 2005 within the sun is hydrogen which is available in at the age of 98, was one of the most influential tremendous volume. Near the centre of the sun, and innovative theoretical physicists of our time. hydrogen nuclei can be squeezed under enormous pressure to become the element helium. If the Bethe was born in Strasbourg, Alsace- mass of hydrogen nucleus can be written as 1, Lorraine (then part of Germany) on 2 July 1906. Bethe showed that each time four nuclei of His father was a University physiologist and his hydrogen fused together as helium, their sum was mother Jewish, the daughter of a Strasbourg not equal to 4. The helium nucleus weighs about professor of Medicine. Bethe moved to Kiel and 0.7% less, or just 3.993 units of weight. It is this Frankfurt from where he went to Munich to carry missing 0.7% that comes out as a burst of explosive out research in theoretical physics under Arnold energy. The sun is so powerful that it pumps 4 Sommerfeld, who was not only a great teacher, million tons of hydrogen into pure energy every but an inspiring research supervisor as well. second. Einstein's famous equation, E = mc2, Bethe received his doctorate in 1928, for the work provides a clue to the massive energy that reaches he did on electron diffraction. He then went to the earth from the sun, when 4 million tons of the Cavendish laboratory in Cambridge on a hydrogen are multiplied by the figure c2 (square Rockefeller Fellowship, and Rome, before of the speed of light). -

Hitler's Uranium Club, the Secret Recordings at Farm Hall
HITLER’S URANIUM CLUB DER FARMHALLER NOBELPREIS-SONG (Melodie: Studio of seiner Reis) Detained since more than half a year Ein jeder weiss, das Unglueck kam Sind Hahn und wir in Farm Hall hier. Infolge splitting von Uran, Und fragt man wer is Schuld daran Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. The real reason nebenbei Die energy macht alles waermer. Ist weil we worked on nuclei. Only die Schweden werden aermer. Und fragt man, wer ist Schuld daran, Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. Die nuclei waren fuer den Krieg Auf akademisches Geheiss Und fuer den allgemeinen Sieg. Kriegt Deutschland einen Nobel-Preis. Und fragt man, wer ist Schuld daran, Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. Wie ist das moeglich, fragt man sich, In Oxford Street, da lebt ein Wesen, The story seems wunderlich. Die wird das heut’ mit Thraenen lesen. Und fragt man, wer ist Schuld daran Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. Die Feldherrn, Staatschefs, Zeitungsknaben, Es fehlte damals nur ein atom, Ihn everyday im Munde haben. Haett er gesagt: I marry you madam. Und fragt man, wer ist Schuld daran, Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. Even the sweethearts in the world(s) Dies ist nur unsre-erste Feier, Sie nennen sich jetzt: “Atom-girls.” Ich glaub die Sache wird noch teuer, Und fragt man, wer ist Schuld daran, Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn.