Grundlagen Mikro- Und Nanosysteme
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Global Transposon Mutagenesis and a Minimal Mycoplasma Genome
R EPORTS thermore, while immunosubunit precursors- stained positive, whereas no staining of PA28Ϫ/Ϫ purchased from PharMingen (San Diego, CA). 25D1.16 containing 15S proteasome assembly inter- cells was observed. (10) and a PA28a-specific antiserum (2) were kindly 11. S. C. Jameson, F. R. Carbone, M. J. Bevan, J. Exp. Med. provided by A. Porgador and M. Rechsteiner, respective- mediates were detected in wild-type cells, 177, 1541 (1993). ly. Metabolic labeling, immunoprecipitation, immuno- under identical conditions 15S complexes 12. T. Preckel and Y. Yang, unpublished results. blotting, and sodium dodecyl sulfateÐpolyacrylamide were hardly detectable in PA28Ϫ/Ϫ cells (Fig. 13. M. Ho, Cytomegalovirus: Biology and Infection (Ple- gel electrophoresis (SDS-PAGE) were performed as de- 4B). Notably, PA28 was found to be associ- num, New York, ed. 2, 1991). scribed (3). 14. P. Borrow and M. B. A. Oldstone, in Viral Pathogenesis, 19. T. Flad et al., Cancer Res. 58, 5803 (1998). ated with the immunosubunit precursor-con- N. Nathanson, Ed. (Lippincott-Raven, Philadelphia, 20. M. W. Moore, F. R. Carbone, M. J. Bevan, Cell 54, 777 taining 15S complexes, suggesting that PA28 1997), pp. 593Ð627; M. J. Buchmeier and A. J. Zajac, (1988). is required for the incorporation of immuno- in Persistent Virus Infections, R. Ahmed and I. Chen, 21. A. Vitiello et al., Eur. J. Immunol. 27, 671 (1997). Eds. ( Wiley, New York, 1999), pp. 575Ð605. subunits into proteasomes. 22. H. Bluthmann et al., Nature 334, 156 (1988). 15. G. Niedermann et al., J. Exp. Med. 186, 209 (1997); 23. W. Jacoby, P. V. Cammarata, S. -

Venter Institute
Genomics:GTL Program Projects J. Craig Venter Institute 42 Estimation of the Minimal Mycoplasma Gene Set Using Global Transposon Mutagenesis and Comparative Genomics John I. Glass* ([email protected]), Nina Alperovich, Nacyra Assad-Garcia, Shibu Yooseph, Mahir Maruf, Carole Lartigue, Cynthia Pfannkoch, Clyde A. Hutchison III, Hamilton O. Smith, and J. Craig Venter J. Craig Venter Institute, Rockville, MD The Venter Institute aspires to make bacteria with specific metabolic capabilities encoded by artificial genomes. To achieve this we must develop technologies and strategies for creating bacterial cells from constituent parts of either biological or synthetic origin. Determining the minimal gene set needed for a functioning bacterial genome in a defined laboratory environment is a necessary step towards our goal. For our initial rationally designed cell we plan to synthesize a genome based on a mycoplasma blueprint (mycoplasma being the common name for the class Mollicutes). We chose this bacterial taxon because its members already have small, near minimal genomes that encode limited metabolic capacity and complexity. We took two approaches to determine what genes would need to be included in a truly minimal synthetic chromosome of a planned Mycoplasma laboratorium: determination of all the non-essential genes through random transposon mutagenesis of model mycoplasma species, Mycoplasma genitalium, and comparative genomics of a set of 15 mycoplasma genomes in order to identify genes common to all members of the taxon. Global transposon mutagenesis has been used to predict the essential gene set for a number of bac- teria. In Bacillus subtilis all but 271 of bacterium’s ~4100 genes could be knocked out. -

Microbial Identification Framework for Risk Assessment
Microbial Identification Framework for Risk Assessment May 2017 Cat. No.: En14-317/2018E-PDF ISBN 978-0-660-24940-7 Information contained in this publication or product may be reproduced, in part or in whole, and by any means, for personal or public non-commercial purposes, without charge or further permission, unless otherwise specified. You are asked to: • Exercise due diligence in ensuring the accuracy of the materials reproduced; • Indicate both the complete title of the materials reproduced, as well as the author organization; and • Indicate that the reproduction is a copy of an official work that is published by the Government of Canada and that the reproduction has not been produced in affiliation with or with the endorsement of the Government of Canada. Commercial reproduction and distribution is prohibited except with written permission from the author. For more information, please contact Environment and Climate Change Canada’s Inquiry Centre at 1-800-668-6767 (in Canada only) or 819-997-2800 or email to [email protected]. © Her Majesty the Queen in Right of Canada, represented by the Minister of the Environment and Climate Change, 2016. Aussi disponible en français Microbial Identification Framework for Risk Assessment Page 2 of 98 Summary The New Substances Notification Regulations (Organisms) (the regulations) of the Canadian Environmental Protection Act, 1999 (CEPA) are organized according to organism type (micro- organisms and organisms other than micro-organisms) and by activity. The Microbial Identification Framework for Risk Assessment (MIFRA) provides guidance on the required information for identifying micro-organisms. This document is intended for those who deal with the technical aspects of information elements or information requirements of the regulations that pertain to identification of a notified micro-organism. -

The Mysterious Orphans of Mycoplasmataceae
The mysterious orphans of Mycoplasmataceae Tatiana V. Tatarinova1,2*, Inna Lysnyansky3, Yuri V. Nikolsky4,5,6, and Alexander Bolshoy7* 1 Children’s Hospital Los Angeles, Keck School of Medicine, University of Southern California, Los Angeles, 90027, California, USA 2 Spatial Science Institute, University of Southern California, Los Angeles, 90089, California, USA 3 Mycoplasma Unit, Division of Avian and Aquatic Diseases, Kimron Veterinary Institute, POB 12, Beit Dagan, 50250, Israel 4 School of Systems Biology, George Mason University, 10900 University Blvd, MSN 5B3, Manassas, VA 20110, USA 5 Biomedical Cluster, Skolkovo Foundation, 4 Lugovaya str., Skolkovo Innovation Centre, Mozhajskij region, Moscow, 143026, Russian Federation 6 Vavilov Institute of General Genetics, Moscow, Russian Federation 7 Department of Evolutionary and Environmental Biology and Institute of Evolution, University of Haifa, Israel 1,2 [email protected] 3 [email protected] 4-6 [email protected] 7 [email protected] 1 Abstract Background: The length of a protein sequence is largely determined by its function, i.e. each functional group is associated with an optimal size. However, comparative genomics revealed that proteins’ length may be affected by additional factors. In 2002 it was shown that in bacterium Escherichia coli and the archaeon Archaeoglobus fulgidus, protein sequences with no homologs are, on average, shorter than those with homologs [1]. Most experts now agree that the length distributions are distinctly different between protein sequences with and without homologs in bacterial and archaeal genomes. In this study, we examine this postulate by a comprehensive analysis of all annotated prokaryotic genomes and focusing on certain exceptions. -

MIB–MIP Is a Mycoplasma System That Captures and Cleaves Immunoglobulin G
MIB–MIP is a mycoplasma system that captures and cleaves immunoglobulin G Yonathan Arfia,b,1, Laetitia Minderc,d, Carmelo Di Primoe,f,g, Aline Le Royh,i,j, Christine Ebelh,i,j, Laurent Coquetk, Stephane Claveroll, Sanjay Vasheem, Joerg Joresn,o, Alain Blancharda,b, and Pascal Sirand-Pugneta,b aINRA (Institut National de la Recherche Agronomique), UMR 1332 Biologie du Fruit et Pathologie, F-33882 Villenave d’Ornon, France; bUniversity of Bordeaux, UMR 1332 Biologie du Fruit et Pathologie, F-33882 Villenave d’Ornon, France; cInstitut Européen de Chimie et Biologie, UMS 3033, University of Bordeaux, 33607 Pessac, France; dInstitut Bergonié, SIRIC BRIO, 33076 Bordeaux, France; eINSERM U1212, ARN Regulation Naturelle et Artificielle, 33607 Pessac, France; fCNRS UMR 5320, ARN Regulation Naturelle et Artificielle, 33607 Pessac, France; gInstitut Européen de Chimie et Biologie, University of Bordeaux, 33607 Pessac, France; hInstitut de Biologie Structurale, University of Grenoble Alpes, F-38044 Grenoble, France; iCNRS, Institut de Biologie Structurale, F-38044 Grenoble, France; jCEA, Institut de Biologie Structurale, F-38044 Grenoble, France; kCNRS UMR 6270, Plateforme PISSARO, Institute for Research and Innovation in Biomedicine - Normandie Rouen, Normandie Université, F-76821 Mont-Saint-Aignan, France; lProteome Platform, Functional Genomic Center of Bordeaux, University of Bordeaux, F-33076 Bordeaux Cedex, France; mJ. Craig Venter Institute, Rockville, MD 20850; nInternational Livestock Research Institute, 00100 Nairobi, Kenya; and oInstitute of Veterinary Bacteriology, University of Bern, CH-3001 Bern, Switzerland Edited by Roy Curtiss III, University of Florida, Gainesville, FL, and approved March 30, 2016 (received for review January 12, 2016) Mycoplasmas are “minimal” bacteria able to infect humans, wildlife, introduced into naive herds (8). -

Role of Protein Phosphorylation in Mycoplasma Pneumoniae
Pathogenicity of a minimal organism: Role of protein phosphorylation in Mycoplasma pneumoniae Dissertation zur Erlangung des mathematisch-naturwissenschaftlichen Doktorgrades „Doctor rerum naturalium“ der Georg-August-Universität Göttingen vorgelegt von Sebastian Schmidl aus Bad Hersfeld Göttingen 2010 Mitglieder des Betreuungsausschusses: Referent: Prof. Dr. Jörg Stülke Koreferent: PD Dr. Michael Hoppert Tag der mündlichen Prüfung: 02.11.2010 “Everything should be made as simple as possible, but not simpler.” (Albert Einstein) Danksagung Zunächst möchte ich mich bei Prof. Dr. Jörg Stülke für die Ermöglichung dieser Doktorarbeit bedanken. Nicht zuletzt durch seine freundliche und engagierte Betreuung hat mir die Zeit viel Freude bereitet. Des Weiteren hat er mir alle Freiheiten zur Verwirklichung meiner eigenen Ideen gelassen, was ich sehr zu schätzen weiß. Für die Übernahme des Korreferates danke ich PD Dr. Michael Hoppert sowie Prof. Dr. Heinz Neumann, PD Dr. Boris Görke, PD Dr. Rolf Daniel und Prof. Dr. Botho Bowien für das Mitwirken im Thesis-Komitee. Der Studienstiftung des deutschen Volkes gilt ein besonderer Dank für die finanzielle Unterstützung dieser Arbeit, durch die es mir unter anderem auch möglich war, an Tagungen in fernen Ländern teilzunehmen. Prof. Dr. Michael Hecker und der Gruppe von Dr. Dörte Becher (Universität Greifswald) danke ich für die freundliche Zusammenarbeit bei der Durchführung von zahlreichen Proteomics-Experimenten. Ein ganz besonderer Dank geht dabei an Katrin Gronau, die mich in die Feinheiten der 2D-Gelelektrophorese eingeführt hat. Außerdem möchte ich mich bei Andreas Otto für die zahlreichen Proteinidentifikationen in den letzten Monaten bedanken. Nicht zu vergessen ist auch meine zweite Außenstelle an der Universität in Barcelona. Dr. Maria Lluch-Senar und Dr. -

Development of an Artificial Cell, from Self- INAUGURAL ARTICLE Organization to Computation and Self-Reproduction
Development of an artificial cell, from self- INAUGURAL ARTICLE organization to computation and self-reproduction Vincent Noireauxa, Yusuke T. Maedab, and Albert Libchaberb,1 aUniversity of Minnesota, 116 Church Street SE, Minneapolis, MN 55455; and bThe Rockefeller University, 1230 York Avenue, New York, NY 10021 This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected in 2007. Contributed by Albert Libchaber, November 22, 2010 (sent for review October 13, 2010) This article describes the state and the development of an artificial the now famous “Omnis cellula e cellula.” Not only is life com- cell project. We discuss the experimental constraints to synthesize posed of cells, but also the most remarkable observation is that a the most elementary cell-sized compartment that can self-reproduce cell originates from a cell and cannot grow in situ. In 1665, Hooke using synthetic genetic information. The original idea was to program made the first observation of cellular organization in cork mate- a phospholipid vesicle with DNA. Based on this idea, it was shown rial (8) (Fig. 1). He also coined the word “cell.” Schleiden later that in vitro gene expression could be carried out inside cell-sized developed a more systematic study (9). The cell model was finally synthetic vesicles. It was also shown that a couple of genes could fully presented by Schwann in 1839 (10). This cellular quantiza- be expressed for a few days inside the vesicles once the exchanges tion was not a priori necessary. Golgi proposed that the branched of nutrients with the outside environment were adequately intro- axons form a continuous network along which the nervous input duced. -

Commentary the Minimal Cell Genome: ''On Being the Right Size”
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 10004–10006, September 1996 Commentary The minimal cell genome: ‘‘On being the right size” Jack Maniloff* Department of Microbiology and Immunology, University of Rochester, Medical Center Box 672, Rochester, NY 14642 ‘‘On being the right size’’ is in quotation marks in the title these studies, it is important to remember that organisms with because it has been used twice before–in each case to discuss small genomes have arisen by two vastly different evolutionary the size of biological systems in terms of interest at that time. pathways, one ‘‘top down’’ and the other ‘‘bottom up.’’ J. B. S. Haldane (1) originally used this title in the 1920s, for ‘‘Top down’’ genomes evolved in organisms with increasing a paper describing the relationship between animal size and metabolic requirements. At each metabolic level, there can be constraints like gravity, surface tension, and food and oxygen a minimal genome: from photoautotrophs, able to grow in a consumption. In the 1970s, N. W. Pirie (2) (acknowledging medium of only CO2, light, and inorganic salts; to simple Haldane) used the title for a paper analyzing how factors like heterotrophs, for which growth requires a basic medium membrane properties, water structure, and the volume needed containing an organic carbon and energy source (like glucose) for ribosomes and other macromolecules set lower limits on and inorganic salts; to fastidious heterotrophs, for which cell size. Now, in the 1990s, the title is used to discuss efforts growth requires a complex medium, frequently containing to define the minimal genome content necessary and sufficient undefined components (like serum); to obligate intracellular for a living cell. -

Synthetic Biology As a Technoscience: the Case of Minimal Genomes and Essential Genes
Published in Studies in History and Philosophy of Science (epub ahead of print) Synthetic Biology as a Technoscience: The Case of Minimal Genomes and Essential Genes Massimiliano Simons, Ghent University [email protected] Department of Philosophy and Moral Sciences, Blandijnberg 2, BE-9000, Ghent Abstract This article examines how minimal genome research mobilizes philosophical concepts such as minimality and essentiality. Following a historical approach the article aims to uncover what function this terminology plays and which problems are raised by them. Specifically, four historical moments are examined, linked to the work of Harold Morowitz, Mitsuhiro Itaya, Eugene Koonin and Arcady Mushegian, and J. Craig Venter. What this survey shows is a historical shift away from historical questions about life or descriptive questions about specific organisms towards questions that explore biological possibilities: what are possible forms of minimal genomes, regardless of whether they exist(ed) in nature? Moreover, it highlights a fundamental ambiguity at work in minimal genome research between a universality claim and a standardization claim: does a minimal genome refer to the minimal gene set for any organism whatsoever? Or does it refer rather to a gene set that will provide stable, robust and predictable behaviour, suited for biotechnological applications? Two diagnoses are proposed for this ambiguity: a philosophical diagnosis of how minimal genome research either misunderstands the ontology of biological entities or philosophically misarticulates scientific practice. Secondly, a historical diagnosis that suggests that this ambiguity is part of a broader shift towards technoscience. Keywords: Minimal genome ; essential gene; Mycoplasma ; Craig Venter; synthetic biology ; technoscience Competing interests No competing interests to declare. -

Identification and Characterization of Mycoplasma Promoters Kevin Lee Knudtson Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1993 Identification and characterization of mycoplasma promoters Kevin Lee Knudtson Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Microbiology Commons, and the Molecular Biology Commons Recommended Citation Knudtson, Kevin Lee, "Identification and characterization of mycoplasma promoters " (1993). Retrospective Theses and Dissertations. 10575. https://lib.dr.iastate.edu/rtd/10575 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. U-M-I MICROFILMED 1994 I INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overlaps. -
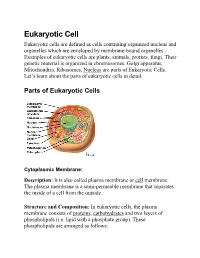
Eukaryotic Cell Eukaryotic Cells Are Defined As Cells Containing Organized Nucleus and Organelles Which Are Enveloped by Membrane-Bound Organelles
Eukaryotic Cell Eukaryotic cells are defined as cells containing organized nucleus and organelles which are enveloped by membrane-bound organelles. Examples of eukaryotic cells are plants, animals, protists, fungi. Their genetic material is organized in chromosomes. Golgi apparatus, Mitochondria, Ribosomes, Nucleus are parts of Eukaryotic Cells. Let’s learn about the parts of eukaryotic cells in detail. Parts ot Eukaryotic Cells Cytoplasmic Membrane: Description: It is also called plasma membrane or cell membrane. The plasma membrane is a semi-permeable membrane that separates the inside of a cell from the outside. Structure and Composition: In eukaryotic cells, the plasma membrane consists of proteins , carbohydrates and two layers of phospholipids (i.e. lipid with a phosphate group). These phospholipids are arranged as follows: • The polar, hydrophilic (water-loving) heads face the outside and inside of the cell. These heads interact with the aqueous environment outside and within a cell. • The non-polar, hydrophobic (water-repelling) tails are sandwiched between the heads and are protected from the aqueous environments. Scientists Singer and Nicolson(1972) described the structure of the phospholipid bilayer as the ‘Fluid Mosaic Model’. The reason is that the bi-layer looks like a mosaic and has a semi-fluid nature that allows lateral movement of proteins within the bilayer. Image: Fluid mosaic model. Orange circles – Hydrophilic heads; Lines below – Hydrophobic tails. Functions • The plasma membrane is selectively permeable i.e. it allows only selected substances to pass through. • It protects the cells from shock and injuries. • The fluid nature of the membrane allows the interaction of molecules within the membrane. -

In Vivo and in Silico Metabolic and Genome Engineering
Landon, S. , Rees, J., Marucci, L., & Grierson, C. (2019). Genome- driven cell engineering review: In vivo and in silico metabolic and genome engineering. Essays in Biochemistry, 63(2), 267-284. https://doi.org/10.1042/EBC20180045 Publisher's PDF, also known as Version of record License (if available): CC BY Link to published version (if available): 10.1042/EBC20180045 Link to publication record in Explore Bristol Research PDF-document University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/red/research-policy/pure/user-guides/ebr-terms/ Essays in Biochemistry (2019) 63 267–284 https://doi.org/10.1042/EBC20180045 Review Article Genome-driven cell engineering review: in vivo and in silico metabolic and genome engineering Sophie Landon1,2,*, Joshua Rees-Garbutt1,3,*, Lucia Marucci1,2,4,† and Claire Grierson1,3,† 1BrisSynBio, University of Bristol, Bristol BS8 1TQ, U.K.; 2Department of Engineering Mathematics, University of Bristol, Bristol BS8 1UB, U.K.; 3School of Biological Sciences, University of Bristol, Life Sciences Building, Bristol BS8 1TQ, U.K.; 4School of Cellular and Molecular Medicine, University of Bristol, Bristol BS8 1UB, U.K. Correspondence: Claire Grierson ([email protected]) or Lucia Marucci ([email protected]) Producing ‘designer cells’ with specific functions is potentially feasible in the near future. Re- cent developments, including whole-cell models, genome design algorithms and gene edit- ing tools, have advanced the possibility of combining biological research and mathematical modelling to further understand and better design cellular processes.