Rapid Detection of Mycoplasma Mycoides Subsp. Capri and Mycoplasma Capricolum Subsp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Deciphering the Spatial Structures of City Networks in the Economic Zone of the West Side of the Taiwan Strait Through the Lens of Functional and Innovation Networks
sustainability Article Deciphering the Spatial Structures of City Networks in the Economic Zone of the West Side of the Taiwan Strait through the Lens of Functional and Innovation Networks Yan Ma * and Feng Xue School of Architecture and Urban-Rural Planning, Fuzhou University, Fuzhou 350108, Fujian, China; [email protected] * Correspondence: [email protected] Received: 17 April 2019; Accepted: 21 May 2019; Published: 24 May 2019 Abstract: Globalization and the spread of information have made city networks more complex. The existing research on city network structures has usually focused on discussions of regional integration. With the development of interconnections among cities, however, the characterization of city network structures on a regional scale is limited in the ability to capture a network’s complexity. To improve this characterization, this study focused on network structures at both regional and local scales. Through the lens of function and innovation, we characterized the city network structure of the Economic Zone of the West Side of the Taiwan Strait through a social network analysis and a Fast Unfolding Community Detection algorithm. We found a significant imbalance in the innovation cooperation among cities in the region. When considering people flow, a multilevel spatial network structure had taken shape. Among cities with strong centrality, Xiamen, Fuzhou, and Whenzhou had a significant spillover effect, which meant the region was depolarizing. Quanzhou and Ganzhou had a significant siphon effect, which was unsustainable. Generally, urbanization in small and midsize cities was common. These findings provide support for government policy making. Keywords: city network; spatial organization; people flows; innovation network 1. -

The Mysterious Orphans of Mycoplasmataceae
The mysterious orphans of Mycoplasmataceae Tatiana V. Tatarinova1,2*, Inna Lysnyansky3, Yuri V. Nikolsky4,5,6, and Alexander Bolshoy7* 1 Children’s Hospital Los Angeles, Keck School of Medicine, University of Southern California, Los Angeles, 90027, California, USA 2 Spatial Science Institute, University of Southern California, Los Angeles, 90089, California, USA 3 Mycoplasma Unit, Division of Avian and Aquatic Diseases, Kimron Veterinary Institute, POB 12, Beit Dagan, 50250, Israel 4 School of Systems Biology, George Mason University, 10900 University Blvd, MSN 5B3, Manassas, VA 20110, USA 5 Biomedical Cluster, Skolkovo Foundation, 4 Lugovaya str., Skolkovo Innovation Centre, Mozhajskij region, Moscow, 143026, Russian Federation 6 Vavilov Institute of General Genetics, Moscow, Russian Federation 7 Department of Evolutionary and Environmental Biology and Institute of Evolution, University of Haifa, Israel 1,2 [email protected] 3 [email protected] 4-6 [email protected] 7 [email protected] 1 Abstract Background: The length of a protein sequence is largely determined by its function, i.e. each functional group is associated with an optimal size. However, comparative genomics revealed that proteins’ length may be affected by additional factors. In 2002 it was shown that in bacterium Escherichia coli and the archaeon Archaeoglobus fulgidus, protein sequences with no homologs are, on average, shorter than those with homologs [1]. Most experts now agree that the length distributions are distinctly different between protein sequences with and without homologs in bacterial and archaeal genomes. In this study, we examine this postulate by a comprehensive analysis of all annotated prokaryotic genomes and focusing on certain exceptions. -

High People's Court of Fujian Province Civil Judgement
High People's Court of Fujian Province Civil Judgement (2015) Min Min Zhong Zi No.2060 Appellant (defendant of the first instance): Xie Zhijin, Male, DOB: 10/27/1963, Han Chinese, Self-employed, residing in Cangshan District, Fuzhou, Fujian Appellant (defendant of the first instance): Ni Mingxiang, Male, DOB: 03/28/1965, Han Chinese, Farmer, residing in Fuqing, Fujian Appellant (defendant of the first instance): Zheng Shijiang, Male, DOB: 04/04/1966, Han Chinese, Farmer, residing in Fuqing, Fujian Attorney of the three appellants above: Xie Changling, Zhong Yin (Fuzhou) Law Firm. Appellee (plaintiff of the first instance): Friends of Nature Domicile: Room A201, Building 2, No. 12 Yumin Road, Chaoyang District, Beijing Legal Representative: Zhang Hehe, Deputy Director-General Entrusted Agent: Ge Feng, Female, Director of Legal and Policy Affairs, residing in Wuchang District, Wuhan Attorney: Liu Xiang, Golden Diamond Law Firm Appellee (plaintiff of the first instance): Fujian Green Home Environment-friendly Center Domicile: 3H, Buidling B, Hot Spring Park, Yingji Road No. 38, Gulou District, Fuzhou, Fujian Legal Representative: Lin Meiying, Director Attorney: Wu Anxin, Hubei Longzhong Law Firm Defendant of the First Instance: Li Mingshuo, Male, DOB: 12/16/1968, Han Chinese, Farmer, residing in Taishun County, Zhejiang Attorney: Qiu Shuhua, Fujian Quanxin Law Firm Third Party of the First Instance: Yanping District Land Resources Bureau of Nanping Municipal Land Resources Bureau Domicile: Shengli Street No. 182, Yanping District, Nanping, Fujian Legal Representative: Huang Ge, Director-General Attorney: He Jianhua, Fujian Shunning Law Firm Third Party of the First Instance: Yanping District Forestry Bureau of Nanping Municipal Forestry Bureau Domicile: Chaoyang Street No. -

Fuzhou, Formerly Romanized As Foochow, Is an Old Port City, Marco Polo Visited It. in the 19Th Century, It Exported More Tea Than Any Other Chinese Port
Fuzhou, formerly Romanized as Foochow, is an old port city, Marco Polo visited it. In the 19th century, it exported more tea than any other Chinese port. Today, it is the capital and one of the largest cities in Fujian province, China. Fuzhou locates in southeast coast of China and is divided from Taiwan Island by Taiwan Strait. Its population was 7.15 million inhabitants, of which urban represents 61.95% while rural population consisting 38.08%. Fuzhou is one of the 14 open costal port cities. The city is also the famous overseas Chinese hometown in China. There are as many as 3 million overseas Chinese originally from Fuzhou, distributing in 102 countries and regions of five continents. Besides Mandarin, Fuzhou region has its own language, called Fuzhou Hua (Fuzhou speech) or Mindong (Eastern Min, where "min" is another name for Fujian). Fuzhou has a humid subtropical climate influenced by the East Asian Monsoon; the summers are long, very hot and humid, and the winters are short, mild and dry. In most years, torrential rain occurs during the monsoon in the second half of May. Fuzhou is also liable to typhoons in late summer and early autumn. The monthly 24-hour average temperature ranges from 10.9 °C (51.6 °F) in January to 28.9 °C (84.0 °F) in July, while the annual mean is 19.84 °C (67.7 °F). With monthly percent possible sunshine ranging from 24 percent in March to 54 percent in July, the city receives 1,607 hours of bright sunshine annually. -

Identification and Characterization of Mycoplasma Promoters Kevin Lee Knudtson Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1993 Identification and characterization of mycoplasma promoters Kevin Lee Knudtson Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Microbiology Commons, and the Molecular Biology Commons Recommended Citation Knudtson, Kevin Lee, "Identification and characterization of mycoplasma promoters " (1993). Retrospective Theses and Dissertations. 10575. https://lib.dr.iastate.edu/rtd/10575 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. U-M-I MICROFILMED 1994 I INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overlaps. -
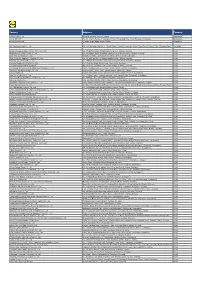
Factory Address Country
Factory Address Country Durable Plastic Ltd. Mulgaon, Kaligonj, Gazipur, Dhaka Bangladesh Lhotse (BD) Ltd. Plot No. 60&61, Sector -3, Karnaphuli Export Processing Zone, North Potenga, Chittagong Bangladesh Bengal Plastics Ltd. Yearpur, Zirabo Bazar, Savar, Dhaka Bangladesh ASF Sporting Goods Co., Ltd. Km 38.5, National Road No. 3, Thlork Village, Chonrok Commune, Korng Pisey District, Konrrg Pisey, Kampong Speu Cambodia Ningbo Zhongyuan Alljoy Fishing Tackle Co., Ltd. No. 416 Binhai Road, Hangzhou Bay New Zone, Ningbo, Zhejiang China Ningbo Energy Power Tools Co., Ltd. No. 50 Dongbei Road, Dongqiao Industrial Zone, Haishu District, Ningbo, Zhejiang China Junhe Pumps Holding Co., Ltd. Wanzhong Villiage, Jishigang Town, Haishu District, Ningbo, Zhejiang China Skybest Electric Appliance (Suzhou) Co., Ltd. No. 18 Hua Hong Street, Suzhou Industrial Park, Suzhou, Jiangsu China Zhejiang Safun Industrial Co., Ltd. No. 7 Mingyuannan Road, Economic Development Zone, Yongkang, Zhejiang China Zhejiang Dingxin Arts&Crafts Co., Ltd. No. 21 Linxian Road, Baishuiyang Town, Linhai, Zhejiang China Zhejiang Natural Outdoor Goods Inc. Xiacao Village, Pingqiao Town, Tiantai County, Taizhou, Zhejiang China Guangdong Xinbao Electrical Appliances Holdings Co., Ltd. South Zhenghe Road, Leliu Town, Shunde District, Foshan, Guangdong China Yangzhou Juli Sports Articles Co., Ltd. Fudong Village, Xiaoji Town, Jiangdu District, Yangzhou, Jiangsu China Eyarn Lighting Ltd. Yaying Gang, Shixi Village, Shishan Town, Nanhai District, Foshan, Guangdong China Lipan Gift & Lighting Co., Ltd. No. 2 Guliao Road 3, Science Industrial Zone, Tangxia Town, Dongguan, Guangdong China Zhan Jiang Kang Nian Rubber Product Co., Ltd. No. 85 Middle Shen Chuan Road, Zhanjiang, Guangdong China Ansen Electronics Co. Ning Tau Administrative District, Qiao Tau Zhen, Dongguan, Guangdong China Changshu Tongrun Auto Accessory Co., Ltd. -

2.18 Fujian Province Fujian Jinghong Group Co., Ltd., Affiliated to The
2.18 Fujian Province Fujian Jinghong Group Co., Ltd., affiliated to the Fujian Provincial Prison Administration Bureau1, has 20 prison entreprises Legal representative of the prison company: Chen Youshun, Chairman of Fujian Jinghong Group Co., Ltd. His official positions in the prison system: Communist Party Committee Deputy Secretary and Political Commissar of Fujian Provincial Prison Administration Bureau2 The Fujian Provincial Prison Administration Bureau has 17 prisons, one juvenile correctional institution, Fujian Jianxin Hospital and the Fujian Provincial Judicial Police Training Corps under its jurisdiction. Business areas: operation and management of state-owned assets of provincial prison enterprises according to the law and under the authorization of the provincial government; production of industrial products, such as mechanical equipment, mold, building materials and cement; processing of clothing, electronic products, footwear and bags; and property management No. Company Name of the Prison, Legal Person Legal representative / Registered Business Scope Company Notes on the Prison Name to which the and Title Capital Address Company Belongs Shareholder(s) 1 Fujian Jinghong Fujian Provincial Prison Fujian Provincial Chen Youshun 833.33 million Operation and 146 Yangqiao The Fujian Provincial Prison Group Co., Ltd. Administration Bureau Prison Chairman of Fujian yuan management of state- Middle Road, Administration Bureau4 is the province’s Administration Jinghong Group Co., Ltd.; owned assets of 10th Floor, penal enforcement -

Federal Register/Vol. 85, No. 156/Wednesday, August 12, 2020
Federal Register / Vol. 85, No. 156 / Wednesday, August 12, 2020 / Notices 48669 does not apply to screen/‘‘surfaced on 4 U.S. Department of Commerce, 1401 investigation as it appeared in the sides’’ (S4S) and/or ‘‘surface 1 side, 2 edges’’ Constitution Avenue NW, Washington, Initiation Notice, as well as additional (SlS2E) stock (also called boards) that are DC 20230; telephone: (202) 482–0768 or language proposed by Commerce. For a finger-jointed, edge-glued mouldings, or (202) 482–1766, respectively. millwork blanks (whether or not resawn). summary of the product coverage Imports of wood mouldings and millwork SUPPLEMENTARY INFORMATION: comments and rebuttal responses products are primarily entered under the Background submitted to the record for this following Harmonized Tariff Schedule of the investigation, and accompanying United States (HTSUS) numbers: This preliminary determination is discussion and analysis of all comments 4409.10.4010, 4409.10.4090, 4409.10.4500, made in accordance with section 733(b) timely received, see the Preliminary 4409.10.5000, 4409.22.4000, 4409.22.5000, of the Tariff Act of 1930, as amended Scope Decision Memorandum.6 4409.29.4100, and 4409.29.5100. Imports of (the Act). Commerce published the wood mouldings and millwork products may notice of initiation of this investigation Commerce is preliminarily modifying also enter under HTSUS numbers: on February 5, 2020.1 On May 26, 2020, the scope language as it appeared in the 4409.10.6000, 4409.10.6500, 4409.22.6000, Commerce postponed the preliminary Initiation Notice. See the revised scope 4409.22.6500, 4409.29.6100, 4409.29.6600, in Appendix I to this notice. -

EANET Site Information
1 Phnom Penh CAMBODIA Site Information Address: Morodok Techo Building (Lot 503) , Tonle Bassac, Chamkarmon, Description: Phnom Penh, Cambodia (All instruments are at the rooftop of MOE, Cambodia.) Code: KHA001 Latitude- Longitude: 11o33‘18"N- 104o56‘20"E Altitude: 12 m Classification: Urban Started: April 2004 Contact Organization: General Directorate of Environmental Protection, Ministry of Environment Monitoring Item 2- - - + + 2+ 2+ + Wet Deposition: Precipitation (pH, EC, SO4 , NO3 , Cl , Na , K , Ca , Mg , NH4 ) Filter pack (SO , HNO , HCl, NH , SO 2-, NO -, Cl-, NH +, Na+, K+, Mg2+, Ca2+ ) Dry Deposition: 2 3 3 4 3 4 Automatic monitor (PM2.5) Monitoring Period Precipitation: Daily Filter pack: Biweekly Automatic monitor: Daily Monitoring Instrument Rain sampler: Eigenbrodt Model D21255 Filter pack sampler: NILU (4 Stages) Automatic monitor: DKK-TOA FRM377-1 (PM2.5) 2 Chongqing- Haifu CHINA Site Information Description: #1, Qingsonglu, Yubei district, Chongqing, China Code: CNA003 Latitude- Longitude: 29°37'30"N- 106°30'34"E Altitude: 300 m Classification: Urban Started: January 2008 Division of Air Quality Monitoring Department, Contact Organization: China National Environmental Monitoring Center Monitoring Item 2- - - + + 2+ 2+ + - - Wet Deposition: Precipitation (pH, EC, SO4 , NO3 , Cl , Na , K , Ca , Mg , NH4 , HCO3 , F ) Monitoring Period Precipitation: Daily Monitoring Instrument Rain sampler: Ogasawara US-320 Chongqing-Jinyunshan CHINA 3 Site Information Description: Dianli conference center, Jinyunshan, Beibei district, -

Deciphering the Spatial Structures of City Networks in the Economic Zone of the West Side of the Taiwan Strait Through the Lens of Functional and Innovation Networks
Article Deciphering the Spatial Structures of City Networks in the Economic Zone of the West Side of the Taiwan Strait Through the Lens of Functional and Innovation Networks Yan Ma 1,* and Feng Xue 2 School of Architecture and Urban Planning, Fuzhou University, Fuzhou 350108, Fujian, China; [email protected] * Correspondence: [email protected] Received: 17 April 2019; Accepted: 21 May 2019; Published: 24 May 2019 Abstract: Globalization and the spread of information have made city networks more complex. The existing research on city network structures has usually focused on discussions of regional integration. With the development of interconnections among cities, however, the characterization of city network structures on a regional scale is limited in the ability to capture a network’s complexity. To improve this characterization, this study focused on network structures at both regional and local scales. Through the lens of function and innovation, we characterized the city network structure of the Economic Zone of the West Side of the Taiwan Strait through a social network analysis and a Fast Unfolding Community Detection algorithm. We found a significant imbalance in the innovation cooperation among cities in the region. When considering people flow, a multilevel spatial network structure had taken shape. Among cities with strong centrality, Xiamen, Fuzhou, and Whenzhou had a significant spillover effect, which meant the region was depolarizing. Quanzhou and Ganzhou had a significant siphon effect, which was unsustainable. Generally, urbanization in small and midsize cities was common. These findings provide support for government policy making. Keywords: city network; spatial organization; people flows; innovation network 1. -

Download This PDF File
2019 International Conference on Energy, Power, Environment and Computer Application (ICEPECA 2019) ISBN: 978-1-60595-612-1 Studies on the Efficiency Characteristics of the Economic Development in Fujian Province Based on DEA and Malmquist Index Hong LEI and Xue-yan HUANG School of Business Administration, Jimei University, Yinjiang Road No. 185, Xiamen, Fujian, P.R. China Keywords: Data envelopment analysis, Economic efficiency, Malmquist productivity Index. Abstract. In this paper, the efficiency characteristics of the economic development of nine cities in Fujian Province are chosen as the research object, collecting 2001-2014 of nine cities in Fujian input-output data, using data envelopment analysis (DEA) combined with Malmquist productivity index, and the technical efficiency, pure technical efficiency and scale efficiency and total factor productivity are calculated. According to the calculation results, the efficiency characteristics of economic development in different areas are analyzed, and the nine cities are divided into three types according to the efficiency characteristics, and the corresponding policy suggestions are proposed. Introduction Fujian province is the starting point of the maritime Silk Road. Since the policy of reform and opening, especially the accession to the WTO, remarkable results have been achieved in the economic development in Fujian; GDP increased from 407 billion Yuan in 2001 to 2405 billion Yuan in 2014, ranking eleventh in the country. At the same time, in the process of economic development, the unbalanced problems in regional development still exist, and the economic development is uneven in efficiency. It is an important problem to analyze the economic development efficiency of various regions in Fujian, to seek the countermeasures for improving the economic efficiency and to promote the balanced development of various regions. -

MIB–MIP Is a Mycoplasma System That Captures and Cleaves Immunoglobulin G
MIB–MIP is a mycoplasma system that captures and cleaves immunoglobulin G Yonathan Arfia,b,1, Laetitia Minderc,d, Carmelo Di Primoe,f,g, Aline Le Royh,i,j, Christine Ebelh,i,j, Laurent Coquetk, Stephane Claveroll, Sanjay Vasheem, Joerg Joresn,o, Alain Blancharda,b, and Pascal Sirand-Pugneta,b aINRA (Institut National de la Recherche Agronomique), UMR 1332 Biologie du Fruit et Pathologie, F-33882 Villenave d’Ornon, France; bUniversity of Bordeaux, UMR 1332 Biologie du Fruit et Pathologie, F-33882 Villenave d’Ornon, France; cInstitut Européen de Chimie et Biologie, UMS 3033, University of Bordeaux, 33607 Pessac, France; dInstitut Bergonié, SIRIC BRIO, 33076 Bordeaux, France; eINSERM U1212, ARN Regulation Naturelle et Artificielle, 33607 Pessac, France; fCNRS UMR 5320, ARN Regulation Naturelle et Artificielle, 33607 Pessac, France; gInstitut Européen de Chimie et Biologie, University of Bordeaux, 33607 Pessac, France; hInstitut de Biologie Structurale, University of Grenoble Alpes, F-38044 Grenoble, France; iCNRS, Institut de Biologie Structurale, F-38044 Grenoble, France; jCEA, Institut de Biologie Structurale, F-38044 Grenoble, France; kCNRS UMR 6270, Plateforme PISSARO, Institute for Research and Innovation in Biomedicine - Normandie Rouen, Normandie Université, F-76821 Mont-Saint-Aignan, France; lProteome Platform, Functional Genomic Center of Bordeaux, University of Bordeaux, F-33076 Bordeaux Cedex, France; mJ. Craig Venter Institute, Rockville, MD 20850; nInternational Livestock Research Institute, 00100 Nairobi, Kenya; and oInstitute of Veterinary Bacteriology, University of Bern, CH-3001 Bern, Switzerland Edited by Roy Curtiss III, University of Florida, Gainesville, FL, and approved March 30, 2016 (received for review January 12, 2016) Mycoplasmas are “minimal” bacteria able to infect humans, wildlife, introduced into naive herds (8).