Mannitol, Picosulphate and Macrogol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Final Stakeholder List PDF 190 KB
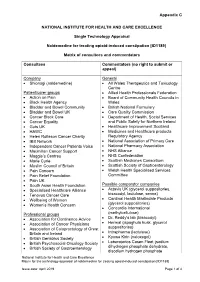
Appendix C NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal Naldemedine for treating opioid-induced constipation [ID1189] Matrix of consultees and commentators Consultees Commentators (no right to submit or appeal) Company General • Shionogi (naldemedine) • All Wales Therapeutics and Toxicology Centre Patient/carer groups • Allied Health Professionals Federation • Action on Pain • Board of Community Health Councils in • Black Health Agency Wales • Bladder and Bowel Community • British National Formulary • Bladder and Bowel UK • Care Quality Commission • Cancer Black Care • Department of Health, Social Services • Cancer Equality and Public Safety for Northern Ireland • Guts UK • Healthcare Improvement Scotland • HAWC • Medicines and Healthcare products • Helen Rollason Cancer Charity Regulatory Agency • IBS Network • National Association of Primary Care • Independent Cancer Patients Voice • National Pharmacy Association • Macmillan Cancer Support • NHS Alliance • Maggie’s Centres • NHS Confederation • Marie Curie • Scottish Medicines Consortium • Muslim Council of Britain • Scottish Society of Gastroenterology • Pain Concern • Welsh Health Specialised Services • Pain Relief Foundation Committee • Pain UK • South Asian Health Foundation Possible comparator companies • Specialised Healthcare Alliance • Actavis UK (glycerol suppositories, • Tenovus Cancer Care bisacodyl, lactulose, senna) • Wellbeing of Women • Cardinal Health Martindale Products • Women’s Health Concern (glycerol suppositories) • Concordia International -

Laxatives for the Management of Constipation in People Receiving Palliative Care (Review)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Laxatives for the management of constipation in people receiving palliative care (Review) Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2015, Issue 5 http://www.thecochranelibrary.com Laxatives for the management of constipation in people receiving palliative care (Review) Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 4 METHODS ...................................... 4 RESULTS....................................... 7 Figure1. ..................................... 8 Figure2. ..................................... 9 Figure3. ..................................... 10 DISCUSSION ..................................... 13 AUTHORS’CONCLUSIONS . 14 ACKNOWLEDGEMENTS . 14 REFERENCES ..................................... 15 CHARACTERISTICSOFSTUDIES . 17 DATAANDANALYSES. 26 ADDITIONALTABLES. 26 APPENDICES ..................................... 28 WHAT’SNEW..................................... 35 HISTORY....................................... 35 CONTRIBUTIONSOFAUTHORS . 36 DECLARATIONSOFINTEREST . 36 SOURCESOFSUPPORT . 36 DIFFERENCES -

3.2.2 Misuse of Stimulant Laxatives
Medicines Adverse Reactions Committee Meeting date 10/06/2021 Agenda item 3.2.2 Title Misuse of stimulant laxatives Submitted by Medsafe Pharmacovigilance Paper type For advice Team Active ingredient Product name Sponsor Bisacodyl Bisacodyl Laxative (Pharmacy Health) PSM Healthcare Limited trading as API tablet Consumer Brands Dulcolax tablet Sanofi-Aventis New Zealand Limited Dulcolax Suppository Sanofi-Aventis New Zealand Limited *Lax-Suppositories Bisacodyl AFT Pharmaceuticals Limited *Lax-Tab tablet AFT Pharmaceuticals Limited Docusate sodium *Coloxyl tablet Pharmacy Retailing (New Zealand) Limited trading as Healthcare Logistics Docusate sodium + Coloxyl with Senna tablet Pharmacy Retailing (New Zealand) sennosides Limited trading as Healthcare Logistics *Laxsol tablet Pharmacy Retailing (New Zealand) Limited trading as Healthcare Logistics Glycerol *Glycerol Suppositories PSM Healthcare Limited trading as API Consumer Brands Sennosides *Senokot tablet Reckitt Benckiser (New Zealand) Limited Sodium picosulfate Dulcolax SP Drops oral solution Sanofi-Aventis New Zealand Limited PHARMAC funding *Pharmaceutical Schedule Lax-Tab tablets, Lax-Suppositories Bisacodyl, Coloxyl tablets and Glycerol Suppositories are fully-funded only on a prescription. Senokot tablets are part- funded. Previous MARC Misuse of stimulant laxatives has not been discussed previously. meetings International action Following a national safety review published in August 2020, the MHRA in the UK has introduced pack size restrictions, revised recommended ages for use -

1: Gastro-Intestinal System
1 1: GASTRO-INTESTINAL SYSTEM Antacids .......................................................... 1 Stimulant laxatives ...................................46 Compound alginate products .................. 3 Docuate sodium .......................................49 Simeticone ................................................... 4 Lactulose ....................................................50 Antimuscarinics .......................................... 5 Macrogols (polyethylene glycols) ..........51 Glycopyrronium .......................................13 Magnesium salts ........................................53 Hyoscine butylbromide ...........................16 Rectal products for constipation ..........55 Hyoscine hydrobromide .........................19 Products for haemorrhoids .................56 Propantheline ............................................21 Pancreatin ...................................................58 Orphenadrine ...........................................23 Prokinetics ..................................................24 Quick Clinical Guides: H2-receptor antagonists .......................27 Death rattle (noisy rattling breathing) 12 Proton pump inhibitors ........................30 Opioid-induced constipation .................42 Loperamide ................................................35 Bowel management in paraplegia Laxatives ......................................................38 and tetraplegia .....................................44 Ispaghula (Psyllium husk) ........................45 ANTACIDS Indications: -

Immediate Hypersensitivity Reactions Caused by Drug Excipients: a Literature Review Caballero ML, Quirce S
REVIEWS Immediate Hypersensitivity Reactions Caused by Drug Excipients: A Literature Review Caballero ML, Quirce S Department of Allergy, La Paz University Hospital, IdiPAZ, Madrid, Spain J Investig Allergol Clin Immunol 2020; Vol. 30(2): 86-100 doi: 10.18176/jiaci.0476 Abstract The European Medicines Agency defines excipients as the constituents of a pharmaceutical form apart from the active substance. Immediate hypersensitivity reactions (IHRs) caused by excipients contained in the formulation of medications have been described. However, there are no data on the prevalence of IHRs due to drug excipients. Clinical manifestations of allergy to excipients can range from skin disorders to life-threatening systemic reactions. The aim of this study was to review the literature on allergy to pharmaceutical excipients and to record the IHRs described with various types of medications, specifically reactions due to the excipients contained in their formulations. The cases reported were sorted alphabetically by type of medication and excipient in order to obtain a list of the excipients most frequently involved for each type of medication. Key words: Allergy. Drug immediate hypersensitivity reaction. Excipient. Pharmaceutical excipients. Resumen La Agencia Europea de Medicamentos define los excipientes como los componentes de una forma farmacéutica diferenciados del principio activo. Se han descrito reacciones de hipersensibilidad inmediata causadas por los excipientes contenidos en la formulación de medicamentos. Sin embargo, no hay datos sobre la prevalencia de dichas reacciones. Las manifestaciones clínicas de la alergia a los excipientes pueden ir desde trastornos de la piel hasta reacciones sistémicas que ponen en peligro la vida. El objetivo de este estudio fue realizar una revisión de la literatura sobre la alergia a los excipientes farmacéuticos y recopilar las reacciones inmediatas descritas con diferentes tipos de medicamento, debido solo a excipientes contenidos en sus formulaciones. -

Prucalopride for the Treatment of Chronic Constipation in Women
Prucalopride for the Treatment of Chronic Constipation in Women: Single Technology Appraisal Response to consultation from Norgine Pharmaceuticals Ltd Comments on clinical assessment 1. The ICER for prucalopride of around £15,000 to £17,000 per QALY gained, whilst probably acceptable for an innovative medicine for a serious or life- threatening condition, is far in excess of what should be considered as acceptable for a laxative. As far as providing value for the NHS is concerned the ICER for Norgine‟s macrogol laxative Movicol is estimated at £250 per QALY gained1, which clearly provides much better value for money. Norgine would therefore question whether prucalopride should be recommended at all by NICE for use in the NHS in England and Wales. 2. If it is considered that prucalopride is cost-effective for use in the NHS in England and Wales, then the preliminary recommendations of the Advisory Committee are not sufficiently precise to avoid doubt as to what the guidance is intended to recommend. For example: (i) It is not clear what is meant by “a clinician with experience of treating chronic constipation.” Many clinicians treat chronic constipation and in numerical terms, nurses probably are involved to a greater extent in managing this condition in primary care than are doctors, and as a result probably have more experience in treating chronic constipation than do primary care physicians. Not all gastroenterologists in secondary care would necessarily qualify as experienced in treating chronic constipation, unless they specialise in the functional bowel disorders. Therefore we would suggest that initially at least the guidance should state that prucalopride therapy should only be initiated by a secondary care physician specialising in the treatment of the functional bowel disorders. -

Oral Lactulose Vs. Polyethylene Glycol for Bowel Preparation in Colonoscopy: a Randomized Controlled Study
Open Access Original Article DOI: 10.7759/cureus.14363 Oral Lactulose vs. Polyethylene Glycol for Bowel Preparation in Colonoscopy: A Randomized Controlled Study Jagdeep Jagdeep 1 , Gaurish Sawant 1 , Pawan Lal 1 , Lovenish Bains 1 1. Department of Surgery, Maulana Azad Medical College, New Delhi, IND Corresponding author: Lovenish Bains, [email protected] Abstract Background Colonoscopy is the method of choice to evaluate colonic mucosa and the distal ileum, allowing the diagnosis and treatment of many diseases. Appropriate bowel preparation necessitates the use of laxative medications, preferentially by oral administration. These include polyethylene glycol (PEG), sodium picosulfate, and sodium phosphate (NaP). Lactulose, a semi-synthetic derivative of lactose, undergoes fermentation, acidifying the gut environment, stimulates intestinal motility, and increases osmotic pressure within the lumen of the colon. Methods In this prospective randomized controlled study, we analyzed 40 patients who presented with symptomatic bleeding per rectum and underwent bowel preparation either with lactulose or polyethylene glycol for colonoscopy. The quality of bowel preparation and other variables like palatability, discomfort, and electrolyte levels were analyzed. Results The majority of the patients (90%) were comfortable with the taste of lactulose solution, whereas the PEG group patients (55%) were equally divided on its palatability. On lactulose consumption, 40% of patients reported nausea/vomiting and around 10% of patients complained of abdominal discomfort. Serum sodium levels showed insignificant changes from 4.33 ± 0.07 mEq/L to 4.21 ± 0.18 mEq/L while potassium also remained similar from 4.26 ± 0.03 mEq/L to 4.22 ± 0.17 mEq/L. The mean Boston Bowel Preparation Score (BBPS) in patients who received lactulose solution was 6.25 ± 0.786 and in those who received PEG solution, it was 6.35 ± 0.813 (P-value = 0.59). -

Drugs Used in Treating Constipation and IBS
Drugs used in treating constipation and IBS Define constipation Know the different symptoms of constipation Know the different lines of treatment of constipation Identify the different types of laxatives Discuss the pharmacokinetics, dynamics, side effects and uses of laxatives Discuss the difference between different treatment including bulk forming laxatives, osmotic laxatives, stimulant laxatives And stool softeners (lubricants). Define bowel syndrome (IBS). Identify the pharmacokinetics, dynamics, side effects and uses of drugs used for IBS. extra information and further explanation important doctors notes Drugs names Mnemonics Kindly check the editing file before studying this document constipation and IBS Very useful. Don’t miss it Infrequent defecation, often with straining and the passage of hard, uncomfortable stools. - May be accompanied by other symptoms: Abdominal discomfort and rectal pain, Flatulence, Loss of appetite, Lethargy & Depression. Decreased motility in colon: Decrease in water and fiber Difficulty in evacuation: Drug-induced: contents of diet. 1. Local painful conditions: 1. Anticholinergic agents “Unbalanced diet” anal fissures, piles. 2. Opioids 2. Lack of muscular 3. Iron exercise. 4. Antipsychotics. 5. Anti-depressant, anti- histamine, has anticholinergic effect Adequate fluid intake High fiber contents in diet Use drugs (laxatives or Regulation of bowel habit. purgatives more watery effect) Regular exercise Avoid drugs causing constipation. • Drugs that hasten the transit of food through the gastrointestinal tract are called (Or we say the drugs that increase GI motility) (محركات) or purgatives(ملينات) laxatives • Loosen stools and increase bowel movement Bulk forming laxatives (increase the size of stool ) • Increase volume of non-absorbable solid residue. Osmotic laxatives (cause water withdrawal by sugar or salt) • Increase water content in large intestine. -

Managing Opioid-Induced Constipation
Visual summary MODIFY PAIN RELIEF Managing opioid-induced constipation Is the current dose of opioid needed to control pain? Constipation affects many patients using opioids Could pain be relieved in other ways? to relieve pain associated with advanced cancer Non-opioid analgesics Interventional pain management or terminal disease. Constipation is often multifactorial in such patients, Could an opioid with less constipating effect be used? and opioids may be one of several causes. As well Buprenorphine Transdermal fentanyl as direct treatment of the constipation, adjusting medication regimens and treating exacerbating factors may help to provide relief from bowel transit Is a referral to other specialists needed? symptoms. Palliative care Neuro-gastroenterology Pain clinic ADDRESS EXACERBATING FACTORS DIRECTLY TREAT CONSTIPATION Drugs Non-pharmacological options 5HT3 antagonists Anticholinergics Opioids Increase fluid consumption Increase dietary fibres Antipsychotics Diuretics Iron Increase physical activity (within patients’ capabilities) Calcium supplements Calcium channel blockers Privacy and comfort during defecation Chemotherapies Complementary therapy Thalidomide Vinca alkaloids Positioning on toilet (to relax the puborectalis muscle) knees higher than hips, leaning forward Busulfan Carboplatin with elbows on knees, straightened spine Manual removal Nutritional and if the constipation is severe and refractory to other therapies metabolic issues Pharmacological management Dehydration Reduced food and fluids intake Oral laxatives Poor -

Treatment of Faecal Impaction in Children Using Combined
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Groningen University of Groningen Treatment of fecal impaction in children using combined polyethylene glycol and sodium picosulphate Lamanna, Anthony; Dughetti, Lauren D.; Jordan-Ely, Julie A.; Dobson, Kyla M.; Dynan, Megan; Foo, Adeline; Kooiman, Louise M. P.; Murakami, Naomi; Fiuza, Kaic; Foroughi, Siavash Published in: BJGP open DOI: 10.1002/jgh3.12062 IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2018 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Lamanna, A., Dughetti, L. D., Jordan-Ely, J. A., Dobson, K. M., Dynan, M., Foo, A., ... Southwell, B. R. (2018). Treatment of fecal impaction in children using combined polyethylene glycol and sodium picosulphate. BJGP open, 2(4), 144-151. https://doi.org/10.1002/jgh3.12062 Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. -

Compound Macrogol Oral Powder Sugar Free. Please Tell Your Doctor Or Pharmacist If You Are Taking, Have Recently Taken Or Might Take Any Other Medicines
Package leaflet: Information for the user Compound Macrogol Oral Powder Sugar Free Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. Always take this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse have told you. - Keep this leaflet. You may need to read it again. - Ask your pharmacist if you need more information or advice. - If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4. - You must talk to a doctor if you do not feel better or if you feel worse after 14 days for chronic constipation, or after 3 days for faecal impaction. What is in this leaflet: 1. What Compound Macrogol Oral Powder Sugar Free is and what it is used for 2. What you need to know before you take Compound Macrogol Oral Powder Sugar Free 3. How to take Compound Macrogol Oral Powder Sugar Free 4. Possible side effects 5. How to store Compound Macrogol Oral Powder Sugar Free 6. Contents of the pack and other information 1. What Compound Macrogol Oral Powder Sugar Free is and what it is used for Compound Macrogol Oral Powder Sugar Free contains macrogol 3350 and the electrolytes sodium chloride, sodium hydrogen carbonate and potassium chloride. Macrogol 3350 is a laxative used for the treatment of constipation, especially if you have been constipated for a long time. It is also used to treat a build up of hard faeces in your bowel which may be a result of long-term constipation (this is known as faecal impaction). -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole