Pc No.136 Medical Use Polyethylene Glycol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemicals Used for Chemical Manufacturing Page 1 of 2
Chemicals used for Chemical Manufacturing Page 1 of 2 Acetic Acid (Glacial, 56%) Glycol Ether PMA Acetone Glycol Ether PNB Acrylic Acid Glycol Ether PNP Activated Carbon Glycol Ether TPM Adipic Acid Glycols Aloe Vera Grease Aluminum Stearate Gum Arabic Aluminum Sulfate Heat Transfer Fluids Amino Acid Heptane Ammonium Acetate Hexane Ammonium Bicarbonate Hydrazine Hydrate Ammonium Bifluoride Hydrochloric Acid (Muriatic) Ammonium Chloride Hydrogen Peroxide Ammonium Citrate Hydroquinone Ammonium Hydroxide Hydroxylamine Sulfate Ammonium Laureth Sulfate Ice Melter Ammonium Lauryl Sulfate Imidazole Ammonium Nitrate Isobutyl Acetate Ammonium Persulfate Isobutyl Alcohol Ammonium Silicofluoride Calcium Stearate Dipropylene Glycol Isopropanolamine Ammonium Sulfate Carboxymethylcellulose Disodium Phosphate Isopropyl Acetate Antifoams Caustic Potash D'Limonene Isopropyl Alcohol Antifreeze Caustic Soda (All Grades) Dodecylbenzene Sulfonic Acid Isopropyl Myristate Antimicrobials Caustic Soda (Beads, Prills) (DDBSA) Isopropyl Palmitate Antimony Oxide Cetyl Alcohol Dowfrost Itaconic Acid Aqua Ammonia Cetyl Palmitate Dowfrost HD Jojoba Oil Ascorbic Acid Chlorine, Granular Dowtherm SR-1 Keratin Barium Carbonate Chloroform Dowtherm 4000 Lactic Acid Barium Chloride Chromic Acid EDTA Lanolin Beeswax Citric Acid (Dry and Liquid) EDTA Plus Lauric Acid Bentonite Coal Epsom Salt Lauryl Alcohol Benzaldehyde Cocamide DEA Ethyl Acetate Lecithin Benzoic Acid Copper Nitrate Ethyl Alcohol (Denatured) Lime Benzyl Alcohol Copper Sulfate Ethylene Glycol Linoleic Acid Bicarbonate -
Final Stakeholder List PDF 190 KB
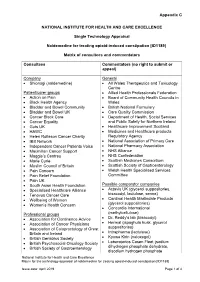
Appendix C NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal Naldemedine for treating opioid-induced constipation [ID1189] Matrix of consultees and commentators Consultees Commentators (no right to submit or appeal) Company General • Shionogi (naldemedine) • All Wales Therapeutics and Toxicology Centre Patient/carer groups • Allied Health Professionals Federation • Action on Pain • Board of Community Health Councils in • Black Health Agency Wales • Bladder and Bowel Community • British National Formulary • Bladder and Bowel UK • Care Quality Commission • Cancer Black Care • Department of Health, Social Services • Cancer Equality and Public Safety for Northern Ireland • Guts UK • Healthcare Improvement Scotland • HAWC • Medicines and Healthcare products • Helen Rollason Cancer Charity Regulatory Agency • IBS Network • National Association of Primary Care • Independent Cancer Patients Voice • National Pharmacy Association • Macmillan Cancer Support • NHS Alliance • Maggie’s Centres • NHS Confederation • Marie Curie • Scottish Medicines Consortium • Muslim Council of Britain • Scottish Society of Gastroenterology • Pain Concern • Welsh Health Specialised Services • Pain Relief Foundation Committee • Pain UK • South Asian Health Foundation Possible comparator companies • Specialised Healthcare Alliance • Actavis UK (glycerol suppositories, • Tenovus Cancer Care bisacodyl, lactulose, senna) • Wellbeing of Women • Cardinal Health Martindale Products • Women’s Health Concern (glycerol suppositories) • Concordia International -

Polyethylene Glycol (PEG) 3350
Polyethylene Glycol (PEG) 3350 Polyethylene glycol is a laxative sold under the trade names MiraLAX®, ClearLax®, GaviLAX®, GlycoLax® and Purelax®. You do not need a doctor’s prescription to buy PEG 3350. It is available over-the-counter. PEG 3350 is an osmotic laxative, which means it increases the water content of stool and is effective in treating or preventing constipation. The body cannot absorb or digest this laxative. PEG 3350 is typically taken once a day by mouth, but the dose may be adjusted higher. It comes as a powder which you will have to mix with liquid. How do I prepare the medication? The standard dose is 17 grams of powder mixed into 8 ounces of liquid. The bottle has a measuring cap that is marked with a line. 1. Pour the powder into the cap up to the marked line. 2. Add the powder in the cap to a full glass (8 ounces) of water, juice, soda, coffee or tea. o If you are over 65, have kidney disease or have liver disease, please only use water. 3. Mix powder well and drink the solution. How do I take PEG 3350? You can take this medication on a full or empty stomach. PEG 3350 doesn’t have any known drug interactions but you should not take other medications at the same time that you take PEG 3350. Other medications may not be digested and absorbed as well. Always drink plenty of decaffeinated liquids with this medication. Michigan Bowel Control Program -1- What are possible side effects? Call your doctor immediately if you have any of the following side-effects: diarrhea severe bloating difficulty breathing painful swelling of the itching of the skin stomach hives vomiting skin rash Other side-effects that usually do not require immediate medical attention are: bloating cramps lower abdominal (stomach) nausea discomfort passing extra gas Some of these symptoms will decrease over time. -

Laxatives for the Management of Constipation in People Receiving Palliative Care (Review)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Laxatives for the management of constipation in people receiving palliative care (Review) Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2015, Issue 5 http://www.thecochranelibrary.com Laxatives for the management of constipation in people receiving palliative care (Review) Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 4 METHODS ...................................... 4 RESULTS....................................... 7 Figure1. ..................................... 8 Figure2. ..................................... 9 Figure3. ..................................... 10 DISCUSSION ..................................... 13 AUTHORS’CONCLUSIONS . 14 ACKNOWLEDGEMENTS . 14 REFERENCES ..................................... 15 CHARACTERISTICSOFSTUDIES . 17 DATAANDANALYSES. 26 ADDITIONALTABLES. 26 APPENDICES ..................................... 28 WHAT’SNEW..................................... 35 HISTORY....................................... 35 CONTRIBUTIONSOFAUTHORS . 36 DECLARATIONSOFINTEREST . 36 SOURCESOFSUPPORT . 36 DIFFERENCES -

Jbscreen Membrane 1 (PEG 400 to PEG 2000 MME Based) Cat.-No.: CS-301L
JBScreen Membrane 1 (PEG 400 to PEG 2000 MME based) Cat.-No.: CS-301L No. Precipitant Precipitant 2 Buffer Additive A1 15 % w/v Polyethylene glycol 400 15 % w/v Glycerol 100 mM HEPES; pH 7.5 200 mM Calcium chloride A2 20 % w/v Polyethylene glycol 400 100 mM Sodium chloride 100 mM tri-Sodium citrate; pH 5.6 20 mM Magnesium chloride A3 25 % w/v Polyethylene glycol 400 none 50 mM Sodium acetate; pH 4.6 50 mM Magnesium acetate A4 30 % w/v Polyethylene glycol 400 50 mM Sodium sulfate 50 mM TRIS; pH 8.5 50 mM Lithium sulfate A5 48 % w/v Polyethylene glycol 400 none 100 mM HEPES; pH 7.5 200 mM Calcium chloride A6 20 % w/v Polyethylene glycol monomethyl ether 550 none 10 mM TRIS; pH 7.5 none A7 30 % w/v Polyethylene glycol monomethyl ether 550 none 50 mM TRIS; pH 8.5 100 mM Magnesium chloride A8 35 % w/v Polyethylene glycol 600 none none none A9 28 % w/v Polyethylene glycol 1,000 10 % w/v Glycerol 100 mM TRICINE; pH 8.0 350 mM Sodium chloride A10 10 % w/v Polyethylene glycol 1,500 5 % w/v Ethanol none 100 mM Magnesium chloride, 100 mM Sodium chloride A11 30 % w/v Polyethylene glycol 1,500 none none none A12 5 % w/v Polyethylene glycol 2,000 none none none B1 10 % w/v Polyethylene glycol 2,000 none 100 mM TRIS; pH 8.5 500 mM Magnesium chloride B2 15 % w/v Polyethylene glycol 2,000 none none none B3 15 % w/v Polyethylene glycol 2,000 none none 100 mM Lithium chloride B4 15 % w/v Polyethylene glycol 2,000 none 100 mM Sodium Phosphate; pH 6.2 20 mM tri-Sodium citrate B5 15 % w/v Polyethylene glycol 2,000 none 100 mM Sodium Phosphate; pH 6.8 500 mM -

Crest ® Pro-Health™ Advanced Sensitive & Enamel Shield
CREST PRO-HEALTH ADVANCED SENSITIVE AND ENAMEL SHIELD- stannous fluoride paste, dentifrice The Procter & Gamble Manufacturing Company Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies. ---------- Crest ® Pro-Health™ Advanced Sensitive & Enamel Shield Drug Facts Active ingredient Stannous fluoride 0.454% (0.16% w/v fluoride ion) Purposes Anticavity, antigingivitis, antisensitivity toothpaste Uses aids in the prevention of cavities helps prevent gingivitis helps interfere with the harmful effects of plaque associated with gingivitis helps control plaque bacteria that contribute to the development of gingivitis builds increasing protection against painful sensitivity of the teeth to cold, heat, acids, sweets or contact Warnings When using this product do not use for sensitivity longer than four weeks unless recommended by a dentist. Stop use and ask a dentist if the sensitivity problem persists or worsens. Sensitive teeth may indicate a serious problem that may need prompt care. Keep out of reach of children. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away. Directions adults and children 12 yrs. & older: apply at least a 1-inch strip of the product onto a soft bristle toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist. Make sure to brush all sensitive -

Polyethylene Glycols: Carefully the Calibration Table of the Molecular Weight Distribution Introduce 25.0 Ml of the Phthalic Anhydride Solution Into a Software
Accessed from 128.83.63.20 by nEwp0rt1 on Tue Nov 29 23:26:06 EST 2011 NF 30 Official Monographs / Polyethylene 1901 pump Mobile phase through the column at this flow rate NMT 112.5% of the labeled nominal value if the labeled for at least 1 h before the first injection. Check the flow nominal value is more than 7000. It may contain a suitable gravimetrically, and adjust it if necessary. Reduce the flow antioxidant. rate to about 0.1 mL/min when the system is not in use.] Injection size: 50 µL ASSAY System suitability • AVERAGE MOLECULAR WEIGHT Sample: Standard solution Phthalic anhydride solution: Place 49.0 g of phthalic anhy- Chromatograph five replicate injections of the Standard dride into an amber bottle, and dissolve in 300 mL of pyri- solution, allowing 15 min between injections, and record dine from a freshly opened bottle or pyridine that has been the retention times of the components of the Standard freshly distilled over phthalic anhydride. Shake vigorously solution. until completely dissolved. Add 7 g of imidazole, swirl care- Insert the average retention time along with the molecular fully to dissolve, and allow to stand for 16 h before using. weight of each component in the Standard solution into Sample solution for liquid Polyethylene Glycols: Carefully the calibration table of the molecular weight distribution introduce 25.0 mL of the Phthalic anhydride solution into a software. Check the regression results for a cubic fit of the dry, heat-resistant pressure bottle. Add an amount of the calibration points, and obtain a correlation coefficient, R, specimen equivalent to its expected average molecular for the line. -

Immediate Hypersensitivity Reactions Caused by Drug Excipients: a Literature Review Caballero ML, Quirce S
REVIEWS Immediate Hypersensitivity Reactions Caused by Drug Excipients: A Literature Review Caballero ML, Quirce S Department of Allergy, La Paz University Hospital, IdiPAZ, Madrid, Spain J Investig Allergol Clin Immunol 2020; Vol. 30(2): 86-100 doi: 10.18176/jiaci.0476 Abstract The European Medicines Agency defines excipients as the constituents of a pharmaceutical form apart from the active substance. Immediate hypersensitivity reactions (IHRs) caused by excipients contained in the formulation of medications have been described. However, there are no data on the prevalence of IHRs due to drug excipients. Clinical manifestations of allergy to excipients can range from skin disorders to life-threatening systemic reactions. The aim of this study was to review the literature on allergy to pharmaceutical excipients and to record the IHRs described with various types of medications, specifically reactions due to the excipients contained in their formulations. The cases reported were sorted alphabetically by type of medication and excipient in order to obtain a list of the excipients most frequently involved for each type of medication. Key words: Allergy. Drug immediate hypersensitivity reaction. Excipient. Pharmaceutical excipients. Resumen La Agencia Europea de Medicamentos define los excipientes como los componentes de una forma farmacéutica diferenciados del principio activo. Se han descrito reacciones de hipersensibilidad inmediata causadas por los excipientes contenidos en la formulación de medicamentos. Sin embargo, no hay datos sobre la prevalencia de dichas reacciones. Las manifestaciones clínicas de la alergia a los excipientes pueden ir desde trastornos de la piel hasta reacciones sistémicas que ponen en peligro la vida. El objetivo de este estudio fue realizar una revisión de la literatura sobre la alergia a los excipientes farmacéuticos y recopilar las reacciones inmediatas descritas con diferentes tipos de medicamento, debido solo a excipientes contenidos en sus formulaciones. -

Prucalopride for the Treatment of Chronic Constipation in Women
Prucalopride for the Treatment of Chronic Constipation in Women: Single Technology Appraisal Response to consultation from Norgine Pharmaceuticals Ltd Comments on clinical assessment 1. The ICER for prucalopride of around £15,000 to £17,000 per QALY gained, whilst probably acceptable for an innovative medicine for a serious or life- threatening condition, is far in excess of what should be considered as acceptable for a laxative. As far as providing value for the NHS is concerned the ICER for Norgine‟s macrogol laxative Movicol is estimated at £250 per QALY gained1, which clearly provides much better value for money. Norgine would therefore question whether prucalopride should be recommended at all by NICE for use in the NHS in England and Wales. 2. If it is considered that prucalopride is cost-effective for use in the NHS in England and Wales, then the preliminary recommendations of the Advisory Committee are not sufficiently precise to avoid doubt as to what the guidance is intended to recommend. For example: (i) It is not clear what is meant by “a clinician with experience of treating chronic constipation.” Many clinicians treat chronic constipation and in numerical terms, nurses probably are involved to a greater extent in managing this condition in primary care than are doctors, and as a result probably have more experience in treating chronic constipation than do primary care physicians. Not all gastroenterologists in secondary care would necessarily qualify as experienced in treating chronic constipation, unless they specialise in the functional bowel disorders. Therefore we would suggest that initially at least the guidance should state that prucalopride therapy should only be initiated by a secondary care physician specialising in the treatment of the functional bowel disorders. -

Amended Safety Assessment of PEG Propylene Glycol Derivatives As Used in Cosmetics
Amended Safety Assessment of PEG Propylene Glycol Derivatives as Used in Cosmetics Status: Final Amended Report Release Date: January 18, 2017 Panel Meeting Date: December 5-6, 2016 The 2016 Cosmetic Ingredient Review Expert Panel members are: Chair, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V. Belsito, M.D.; Ronald A. Hill, Ph.D.; Curtis D. Klaassen, Ph.D.; Daniel C. Liebler, Ph.D.; James G. Marks, Jr., M.D.; Ronald C. Shank, Ph.D.; Thomas J. Slaga, Ph.D.; and Paul W. Snyder, D.V.M., Ph.D. The CIR Director is Lillian J. Gill, D.P.A. This report was prepared by Lillian C. Becker, Scientific Analyst/Writer. © Cosmetic Ingredient Review 1620 L Street, NW, Suite 1200 Washington, DC 20036-4702 ph 202.331.0651 fax 202.331.0088 [email protected] ABSTRACT This is an amended safety assessment of PEG propylene glycol derivatives as used in cosmetics. These seven ingredients mostly function as surfactants and skin-conditioning agents. The Cosmetic Ingredient Review (CIR) Expert Panel (Panel) reviewed relevant data related to these ingredients. Because there were little data on these ingredients, the Panel relied on other CIR reports on related ingredients, the moieties, and component parts of these ingredients for read across and informational purposes. The Panel agreed that the caveat from the previous safety assessment, i.e., that ingredients containing PEGs should not be used on damaged skin, should be removed. The Panel concluded that these PEG propylene glycol derivatives are safe in cosmetics in the present practices of use and concentration described in this safety assessment. -

Drugs Used in Treating Constipation and IBS
Drugs used in treating constipation and IBS Define constipation Know the different symptoms of constipation Know the different lines of treatment of constipation Identify the different types of laxatives Discuss the pharmacokinetics, dynamics, side effects and uses of laxatives Discuss the difference between different treatment including bulk forming laxatives, osmotic laxatives, stimulant laxatives And stool softeners (lubricants). Define bowel syndrome (IBS). Identify the pharmacokinetics, dynamics, side effects and uses of drugs used for IBS. extra information and further explanation important doctors notes Drugs names Mnemonics Kindly check the editing file before studying this document constipation and IBS Very useful. Don’t miss it Infrequent defecation, often with straining and the passage of hard, uncomfortable stools. - May be accompanied by other symptoms: Abdominal discomfort and rectal pain, Flatulence, Loss of appetite, Lethargy & Depression. Decreased motility in colon: Decrease in water and fiber Difficulty in evacuation: Drug-induced: contents of diet. 1. Local painful conditions: 1. Anticholinergic agents “Unbalanced diet” anal fissures, piles. 2. Opioids 2. Lack of muscular 3. Iron exercise. 4. Antipsychotics. 5. Anti-depressant, anti- histamine, has anticholinergic effect Adequate fluid intake High fiber contents in diet Use drugs (laxatives or Regulation of bowel habit. purgatives more watery effect) Regular exercise Avoid drugs causing constipation. • Drugs that hasten the transit of food through the gastrointestinal tract are called (Or we say the drugs that increase GI motility) (محركات) or purgatives(ملينات) laxatives • Loosen stools and increase bowel movement Bulk forming laxatives (increase the size of stool ) • Increase volume of non-absorbable solid residue. Osmotic laxatives (cause water withdrawal by sugar or salt) • Increase water content in large intestine. -

In Vitro Effect of Polyethylene Glycol and Sorbitol on Two Banana Varieties Viz
Published online: May 9. 2021 ISSN : 0974-9411 (Print), 2231-5209 (Online) journals.ansfoundation.org Research Article In vitro effect of polyethylene glycol and sorbitol on two banana varieties viz. Grand naine and Nalla bontha to study drought stress Ashu Singh Department of Agricultural Biotechnology, College of Agriculture, Sardar Vallabhbhai Patel Article Info University of Agriculture and Technology, Meerut-250110 (U.P.), India https://doi.org/10.31018/ Pradeep Kumar Shukla jans.v13i2.2579 Department of Biological Sciences, Faculty of Basic Sciences, Sam Higginbottom University Received: February 19, 2021 of Agriculture, Technology and Sciences, Prayagraj-211007 (U.P.), India Revised: April 30, 2021 Accepted: May 7, 2021 R. S. Sengar Department of Agricultural Biotechnology, College of Agriculture, Sardar Vallabhbhai Patel University of Agriculture and Technology, Meerut-250110 (U.P.), India Pragati Mishra* Department of Molecular and Cellular Engineering, Jacob Institute of Biotechnology and Bioengineering, Sam Higginbottom University of Agriculture, Technology and Sciences, Prayagraj-211007 (U.P.), India *Corresponding author. Email: [email protected] How to Cite Singh, A. et al. (2021). In vitro effect of polyethylene glycol and sorbitol on two banana varieties viz. Grand naine and Nalla bontha to study drought stress. Journal of Applied and Natural Science, 13(2), 482 - 490. https://doi.org/10.31018/jans.v13i2.2579 Abstract Water stress is one of the foremost categories of stress damaging plants’ overall growth and development. The aim of the present study was to explore and demonstrate stress-induced drought to calibrate changes in stress parameters of two banana plant varieties viz. Grand naine (G9) and Nalla bontha were cultured in Murashige and Skoog medium (MS) media supple- mented with stress inducers -Poly ethylene glycol (PEG) and sorbitol.