Laxatives for the Management of Constipation in People Receiving Palliative Care (Review)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemicals Used for Chemical Manufacturing Page 1 of 2
Chemicals used for Chemical Manufacturing Page 1 of 2 Acetic Acid (Glacial, 56%) Glycol Ether PMA Acetone Glycol Ether PNB Acrylic Acid Glycol Ether PNP Activated Carbon Glycol Ether TPM Adipic Acid Glycols Aloe Vera Grease Aluminum Stearate Gum Arabic Aluminum Sulfate Heat Transfer Fluids Amino Acid Heptane Ammonium Acetate Hexane Ammonium Bicarbonate Hydrazine Hydrate Ammonium Bifluoride Hydrochloric Acid (Muriatic) Ammonium Chloride Hydrogen Peroxide Ammonium Citrate Hydroquinone Ammonium Hydroxide Hydroxylamine Sulfate Ammonium Laureth Sulfate Ice Melter Ammonium Lauryl Sulfate Imidazole Ammonium Nitrate Isobutyl Acetate Ammonium Persulfate Isobutyl Alcohol Ammonium Silicofluoride Calcium Stearate Dipropylene Glycol Isopropanolamine Ammonium Sulfate Carboxymethylcellulose Disodium Phosphate Isopropyl Acetate Antifoams Caustic Potash D'Limonene Isopropyl Alcohol Antifreeze Caustic Soda (All Grades) Dodecylbenzene Sulfonic Acid Isopropyl Myristate Antimicrobials Caustic Soda (Beads, Prills) (DDBSA) Isopropyl Palmitate Antimony Oxide Cetyl Alcohol Dowfrost Itaconic Acid Aqua Ammonia Cetyl Palmitate Dowfrost HD Jojoba Oil Ascorbic Acid Chlorine, Granular Dowtherm SR-1 Keratin Barium Carbonate Chloroform Dowtherm 4000 Lactic Acid Barium Chloride Chromic Acid EDTA Lanolin Beeswax Citric Acid (Dry and Liquid) EDTA Plus Lauric Acid Bentonite Coal Epsom Salt Lauryl Alcohol Benzaldehyde Cocamide DEA Ethyl Acetate Lecithin Benzoic Acid Copper Nitrate Ethyl Alcohol (Denatured) Lime Benzyl Alcohol Copper Sulfate Ethylene Glycol Linoleic Acid Bicarbonate -
Final Stakeholder List PDF 190 KB
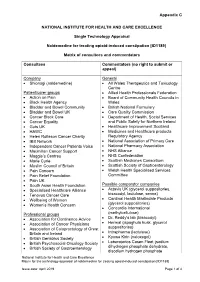
Appendix C NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal Naldemedine for treating opioid-induced constipation [ID1189] Matrix of consultees and commentators Consultees Commentators (no right to submit or appeal) Company General • Shionogi (naldemedine) • All Wales Therapeutics and Toxicology Centre Patient/carer groups • Allied Health Professionals Federation • Action on Pain • Board of Community Health Councils in • Black Health Agency Wales • Bladder and Bowel Community • British National Formulary • Bladder and Bowel UK • Care Quality Commission • Cancer Black Care • Department of Health, Social Services • Cancer Equality and Public Safety for Northern Ireland • Guts UK • Healthcare Improvement Scotland • HAWC • Medicines and Healthcare products • Helen Rollason Cancer Charity Regulatory Agency • IBS Network • National Association of Primary Care • Independent Cancer Patients Voice • National Pharmacy Association • Macmillan Cancer Support • NHS Alliance • Maggie’s Centres • NHS Confederation • Marie Curie • Scottish Medicines Consortium • Muslim Council of Britain • Scottish Society of Gastroenterology • Pain Concern • Welsh Health Specialised Services • Pain Relief Foundation Committee • Pain UK • South Asian Health Foundation Possible comparator companies • Specialised Healthcare Alliance • Actavis UK (glycerol suppositories, • Tenovus Cancer Care bisacodyl, lactulose, senna) • Wellbeing of Women • Cardinal Health Martindale Products • Women’s Health Concern (glycerol suppositories) • Concordia International -

Potassium-Magnesium Citrate Is an Effective Prophylaxis Against Recurrent Calcium Oxalate Nephrolithiasis
0022-5347/97/1586-2069$03.00/0 JOURNAL OF UROLOGY Vol. 158,2069-2073, December 1997 Copyright Q 1997 by AMERICANUROLOGICAL ASS~CIATION, INC. Printed in U.S.A. POTASSIUM-MAGNESIUM CITRATE IS AN EFFECTIVE PROPHYLAXIS AGAINST RECURRENT CALCIUM OXALATE NEPHROLITHIASIS BRUCE ETTINGER,* CHARLES Y. C. PAK, JOHN T. CITRON, CARL THOMAS, BEVERLEY ADAMS-HIJET AND ARLINE VANGESSEL From the Diuision of Research, Kaiser Permanente Medical Care Program, Oakland, California, the Department of Mineral Metabolism, Center for Mineral Metabolism and Clinical Research, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas, Department of Medicine, Kaiser Permanente Medical Center, Walnut Creek, California, Department of Urology, Kaiser Permanente Medical Center, San Francisco, California, and Kaiser Foundation Research Institute, Kaiser Foundation Hospitals, Oakland, California ABSTRACT Purpose: We examined the efficacy of potassium-magnesium citrate in preventing recurrent calcium oxalate kidney calculi. Materials and Methods: We conducted a prospective double-blind study of 64 patients who were randomly assigned to receive placebo or potassium-magnesium citrate (42 mEq. potassium, 21 mEq. magnesium, and 63 mEq. citrate) daily for up to 3 years. Results. New calculi formed in 63.6%of subjects receiving placebo and in 12.9%of subjects receiving potassium-magnesiumcitrate. When compared with placebo, the relative risk of treat- ment failure for potassium-magnesium citrate was 0.16 (95%confidence interval 0.05 to 0.46). potassium-magnesium citrate had a statistically significant effect (relative risk 0.10,95%confi- dence interval 0.03 to 0.36) even after adjustment for possible confounders, including age, pretreatment calculous event rate and urinary biochemical abnormalities. -

Peptide Therapeutics Designing a Science-Led Strategic Quality Control Program
BioProcess International Peptide SPECIAL REPORT Therapeutics Designing a Science-Led Strategic Quality Control Program INTERTEK PHARMACEUTICAL SERVICES Your partner for regulatory-driven, phase appropriate analytical programs tailored to your molecule. Our experts help you to navigate the challenges of development, regulatory submission, and manufacturing. Peptide Therapeutics Designing a Science-Led Strategic Quality Control Program Shashank Sharma and Hannah Lee ince the emergence of peptide therapeutics in the 1920s with the advent of insulin therapy, the market for this product class has continued to expand with global revenues anticipatedS to surpass US$50 billion by 2024 (1). The growth of peptide therapeutics is attributed not only to improvements in manufacturing, but also to a rise in demand because of an increasingly aging population that is driving an increase in the occurrence of long-term diseases. The need for efficient and low-cost drugs and rising investments in research and development of novel drugs continues to boost market growth and fuel the emergence of generic versions that offer patients access to vital medicines at low costs. North America has been the dominant market for peptide therapeutics, with the Asia–Pacific region Insulin molecular model; the first therapeutic expected to grow at a faster rate. The global peptides use of this peptide hormone was in the market has attracted the attention of key players 1920s to treat diabetic patients. within the pharmaceutical industry, including Teva Pharmaceuticals, Eli Lilly, Novo Nordisk, Pfizer, amino acids to be peptides. Within that set, those Takeda, and Amgen. Those companies have made containing 10 or more are classed as polypeptides. -

Linaclotide: a Novel Therapy for Chronic Constipation and Constipation- Predominant Irritable Bowel Syndrome Brian E
Linaclotide: A Novel Therapy for Chronic Constipation and Constipation- Predominant Irritable Bowel Syndrome Brian E. Lacy, PhD, MD, John M. Levenick, MD, and Michael D. Crowell, PhD, FACG Dr. Lacy is Section Chief of Gastroenter- Abstract: Chronic constipation and irritable bowel syndrome ology and Hepatology and Dr. Levenick (IBS) are functional gastrointestinal disorders that significantly is a Gastroenterology Fellow in the affect patients’ quality of life. Chronic constipation and IBS are Division of Gastroenterology and prevalent—12% of the US population meet the diagnostic crite- Hepatology at Dartmouth-Hitchcock Medical Center in Lebanon, New ria for IBS, and 15% meet the criteria for chronic constipation— Hampshire. Dr. Crowell is a Professor and these conditions negatively impact the healthcare system of Medicine in the Division of from an economic perspective. Despite attempts at dietary Gastroenterology and Hepatology at modification, exercise, or use of over-the-counter medications, Mayo Clinic in Scottsdale, Arizona. many patients have persistent symptoms. Alternative treatment options are limited. This article describes linaclotide (Linzess, Address correspondence to: Dr. Brian E. Lacy Ironwood Pharmaceuticals/Forest Pharmaceuticals), a new, first- Division of Gastroenterology and in-class medication for the treatment of chronic constipation Hepatology, Area 4C and constipation-predominant IBS. Dartmouth-Hitchcock Medical Center 1 Medical Center Drive Lebanon, NH 03756; Tel: 603-650-5215; Fax: 603-650-5225; onstipation is -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -
Managing Constipation in Adults
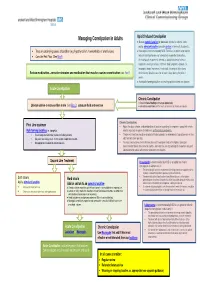
Managing Constipation in Adults Opio id Induced Constipation ● Use an osmotic laxative (or docusate which also softens stools and a stimulant laxative (consider dantron in terminally ill patients). • Treat any underlying causes of condition (e.g. hypothyroidism, haemorrhoids or anal fissures) ● Naloxegol is recommended by NICE TA345 as an option when opioid induced constipation has not adequately responded to laxatives. • Consider Red Flags (See Box A ) An inadequate response is defined as opioid-induced of at least moderate severity in at least 1of the 4 stool symptoms domain (i.e. incomplete bowel movement, hard stool, straining or false alarm) Review medication –consider stopping any medication that may be causing constipation (see Box B ) whilst taking1 laxative class for at least 4 days during the prior 2 weeks. ● Avoid bulk -forming l axatives for treating opioid induced constipation Acute Constipation Chronic Constipation ● Check for faecal loading and manage appropriately Lifestyle advise -to increase fibre in diet (see Box C ), adequate fluid and exercise ● Set realistic expectations for the results of treatment of chronic constipation. Chronic Constipation: First Line treatment • Adjust the dose, choice, and combination of laxative according to symptoms, speed with which Bulk forming laxatives i.e. ispaghula. relief is required, response to treatment, and individual preference. • Ensure adequate fluid intake (caution- frail elderly patients) • The dose of laxative should be gradually titrated upwards (or downwards) to produce one or two • May take several days to act- so not suitable if rapid relief required. soft, formed stools per day. • Not appropriate for opiod induced constipation. • If at least two laxatives (from different classes) have been tried at the highest tolerated recommended doses for at least 6 months, consider the use of prucalopride in women only and lubiprostone for adults with chronic idiopathic constipation. -
Acid Reflux and Gastroesophageal Reflux Disease in Adults (The Text Basics) Graphics
Official reprint from UpToDate® www.uptodate.com ©2020 UpToDate, Inc. and/or its affiliates. All Rights Reserved. Print Options Print | Back English Patient education: Acid reflux and gastroesophageal reflux disease in adults (The Text Basics) Graphics Written by the doctors and editors at UpToDate What is acid reflux? Acid reflux is when the acid that is normally in your stomach backs up into the esophagus. The esophagus is the tube that carries food from your mouth to your stomach (figure 1). When acid reflux causes bothersome symptoms or damage, doctors call it "gastroesophageal reflux disease" or "GERD." What are the symptoms of acid reflux? The most common symptoms are: ● Heartburn, which is a burning feeling in the chest ● Regurgitation, which is when acid and undigested food flow back into your throat or mouth Other symptoms might include: ● Stomach or chest pain ● Trouble swallowing ● Having a raspy voice or a sore throat ● Unexplained cough ● Nausea or vomiting Is there anything I can do on my own to feel better? Yes. You might feel better if you: ● Lose weight (if you are overweight) ● Raise the head of your bed by 6 to 8 inches – You can do this by putting blocks of wood or rubber under 2 legs of the bed or a foam wedge under the mattress. ● Avoid foods that make your symptoms worse – For some people these include coffee, chocolate, alcohol, peppermint, and fatty foods. ● Stop smoking, if you smoke ● Avoid late meals – Lying down with a full stomach can make reflux worse. Try to plan meals for at least 2 to 3 hours before bedtime. -

COMPASS Therapeutic Notes on the Management of Chronic Constipation in Primary Care
COMPASS Therapeutic Notes on the Management of Chronic Constipation in Primary Care In this issue: Glossary Page Chronic Constipation lasting longer than 3 months Introduction and background 1 constipation Drugs used in the management of Functional Chronic constipation without a known cause. Also known as primary 3 chronic constipation constipation constipation or idiopathic constipation Bulk-forming laxatives 4 Gastrocolic The occurrence of peristalsis following the entrance of food into the Osmotic laxatives 5 response empty stomach Stimulant laxatives 6 IBS Irritable Bowel Syndrome IBS-C Irritable Bowel Syndrome with Constipation Faecal softeners 7 Melanosis Dark brownish black pigmentation of the mucous membrane of the colon Peripheral opioid-receptor 7 coli due to the deposition of pigment in macrophages antagonists Myenteric Part of the enteric nervous system with an important role in regulating 5HT4 – receptor agonists 7 plexus gut motility Managing constipation in 8 NNT Number Needed to Treat pregnancy and breastfeeding OTC Over The Counter RCT Randomised Controlled Trial Secondary Constipation caused by a drug or medical condition. Secondary constipation constipation is also known as organic constipation SmPC Summary of Product Characteristics A twisting or looping of the bowel resulting in obstruction; can be life- Volvulus threatening Successful completion of the assessment questions at the end of this issue will provide you with 2 hours towards your CPD/CME requirements. Further copies of this and any other edition in the -

3.2.2 Misuse of Stimulant Laxatives
Medicines Adverse Reactions Committee Meeting date 10/06/2021 Agenda item 3.2.2 Title Misuse of stimulant laxatives Submitted by Medsafe Pharmacovigilance Paper type For advice Team Active ingredient Product name Sponsor Bisacodyl Bisacodyl Laxative (Pharmacy Health) PSM Healthcare Limited trading as API tablet Consumer Brands Dulcolax tablet Sanofi-Aventis New Zealand Limited Dulcolax Suppository Sanofi-Aventis New Zealand Limited *Lax-Suppositories Bisacodyl AFT Pharmaceuticals Limited *Lax-Tab tablet AFT Pharmaceuticals Limited Docusate sodium *Coloxyl tablet Pharmacy Retailing (New Zealand) Limited trading as Healthcare Logistics Docusate sodium + Coloxyl with Senna tablet Pharmacy Retailing (New Zealand) sennosides Limited trading as Healthcare Logistics *Laxsol tablet Pharmacy Retailing (New Zealand) Limited trading as Healthcare Logistics Glycerol *Glycerol Suppositories PSM Healthcare Limited trading as API Consumer Brands Sennosides *Senokot tablet Reckitt Benckiser (New Zealand) Limited Sodium picosulfate Dulcolax SP Drops oral solution Sanofi-Aventis New Zealand Limited PHARMAC funding *Pharmaceutical Schedule Lax-Tab tablets, Lax-Suppositories Bisacodyl, Coloxyl tablets and Glycerol Suppositories are fully-funded only on a prescription. Senokot tablets are part- funded. Previous MARC Misuse of stimulant laxatives has not been discussed previously. meetings International action Following a national safety review published in August 2020, the MHRA in the UK has introduced pack size restrictions, revised recommended ages for use -

1: Gastro-Intestinal System
1 1: GASTRO-INTESTINAL SYSTEM Antacids .......................................................... 1 Stimulant laxatives ...................................46 Compound alginate products .................. 3 Docuate sodium .......................................49 Simeticone ................................................... 4 Lactulose ....................................................50 Antimuscarinics .......................................... 5 Macrogols (polyethylene glycols) ..........51 Glycopyrronium .......................................13 Magnesium salts ........................................53 Hyoscine butylbromide ...........................16 Rectal products for constipation ..........55 Hyoscine hydrobromide .........................19 Products for haemorrhoids .................56 Propantheline ............................................21 Pancreatin ...................................................58 Orphenadrine ...........................................23 Prokinetics ..................................................24 Quick Clinical Guides: H2-receptor antagonists .......................27 Death rattle (noisy rattling breathing) 12 Proton pump inhibitors ........................30 Opioid-induced constipation .................42 Loperamide ................................................35 Bowel management in paraplegia Laxatives ......................................................38 and tetraplegia .....................................44 Ispaghula (Psyllium husk) ........................45 ANTACIDS Indications: -

Gastroesophageal Reflux Disease (GERD)
Guidelines for Clinical Care Quality Department Ambulatory GERD Gastroesophageal Reflux Disease (GERD) Guideline Team Team Leader Patient population: Adults Joel J Heidelbaugh, MD Objective: To implement a cost-effective and evidence-based strategy for the diagnosis and Family Medicine treatment of gastroesophageal reflux disease (GERD). Team Members Key Points: R Van Harrison, PhD Diagnosis Learning Health Sciences Mark A McQuillan, MD History. If classic symptoms of heartburn and acid regurgitation dominate a patient’s history, then General Medicine they can help establish the diagnosis of GERD with sufficiently high specificity, although sensitivity Timothy T Nostrant, MD remains low compared to 24-hour pH monitoring. The presence of atypical symptoms (Table 1), Gastroenterology although common, cannot sufficiently support the clinical diagnosis of GERD [B*]. Testing. No gold standard exists for the diagnosis of GERD [A*]. Although 24-hour pH monitoring Initial Release is accepted as the standard with a sensitivity of 85% and specificity of 95%, false positives and false March 2002 negatives still exist [II B*]. Endoscopy lacks sensitivity in determining pathologic reflux but can Most Recent Major Update identify complications (eg, strictures, erosive esophagitis, Barrett’s esophagus) [I A]. Barium May 2012 radiography has limited usefulness in the diagnosis of GERD and is not recommended [III B*]. Content Reviewed Therapeutic trial. An empiric trial of anti-secretory therapy can identify patients with GERD who March 2018 lack alarm or warning symptoms (Table 2) [I A*] and may be helpful in the evaluation of those with atypical manifestations of GERD, specifically non-cardiac chest pain [II B*]. Treatment Ambulatory Clinical Lifestyle modifications.