Drugs Used in Treating Constipation and IBS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplemental
Supporting Information Aqueous Multiphase Systems of Polymers and Surfactants Provide Self-Assembling Gradients in Density Charles R. Mace1, Ozge Akbulut1, Ashok A. Kumar2, Nathan D. Shapiro1, Ratmir Derda1, Matthew R. Patton1, and George M. Whitesides1,3* 1Department of Chemistry & Chemical Biology, Harvard University, 12 Oxford St., Cambridge, MA 02138, United States, 2School of Engineering and Applied Sciences, Harvard University, 29 Oxford St., Cambridge, MA 02138, United States, and 3Wyss Institute for Biologically Inspired Engineering, Harvard University, 60 Oxford St., Cambridge, MA 02138, United States *Corresponding author: [email protected] S1 Materials and Methods Materials. The following chemicals were purchased from Sigma-Aldrich: alginic acid sodium salt, chondroitin sulfate A, dextran sulfate sodium salt, Ficoll, (hydroxypropyl)methyl cellulose, poly(2-acrylamido-2-methyl-1-propanesulfonic acid), poly(2-ethyl-2-oxazoline), polyacrylamide, poly(diallyldimethylammonium chloride), poly(ethylene glycol), polyethyleneimine, poly(methacrylic acid sodium salt), poly(propylene glycol), polyvinylpyrrolidone, Brij 35, 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), cetyl trimethylammonium bromide (CTAB), Pluronic F68, sodium chloate, Tween 20, Triton X-100, Zonyl, lithium bromide (LiBr), cesium bromide (CsBr) and benzene-1,2-disulfonic acid dipotassium salt. The following chemicals were purchased from Polysciences: poly(acrylic acid), poly(allylamine hydrochloride), poly(styrenesulfonic acid sodium salt), and poly(vinyl alcohol). Dextran was purchased from Spectrum Chemical. Diethylaminoethyl-dextran hydrochloride was purchased from MP Biomedicals. Carboxy-modified polyacrylamide, hydroxyethyl cellulose, methyl cellulose were purchased from Scientific Polymer Products. N- octyl-β-D-glucopyranoside was purchased from Calbiochem. N,N-dimethyldodecylamine N- oxide was purchased from Fluka. Sodium dodecyl sulfate was purchased from J.T. -
Final Stakeholder List PDF 190 KB
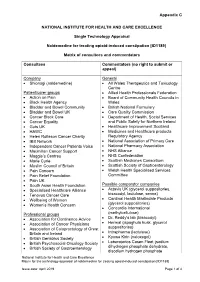
Appendix C NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal Naldemedine for treating opioid-induced constipation [ID1189] Matrix of consultees and commentators Consultees Commentators (no right to submit or appeal) Company General • Shionogi (naldemedine) • All Wales Therapeutics and Toxicology Centre Patient/carer groups • Allied Health Professionals Federation • Action on Pain • Board of Community Health Councils in • Black Health Agency Wales • Bladder and Bowel Community • British National Formulary • Bladder and Bowel UK • Care Quality Commission • Cancer Black Care • Department of Health, Social Services • Cancer Equality and Public Safety for Northern Ireland • Guts UK • Healthcare Improvement Scotland • HAWC • Medicines and Healthcare products • Helen Rollason Cancer Charity Regulatory Agency • IBS Network • National Association of Primary Care • Independent Cancer Patients Voice • National Pharmacy Association • Macmillan Cancer Support • NHS Alliance • Maggie’s Centres • NHS Confederation • Marie Curie • Scottish Medicines Consortium • Muslim Council of Britain • Scottish Society of Gastroenterology • Pain Concern • Welsh Health Specialised Services • Pain Relief Foundation Committee • Pain UK • South Asian Health Foundation Possible comparator companies • Specialised Healthcare Alliance • Actavis UK (glycerol suppositories, • Tenovus Cancer Care bisacodyl, lactulose, senna) • Wellbeing of Women • Cardinal Health Martindale Products • Women’s Health Concern (glycerol suppositories) • Concordia International -

Laxatives for the Management of Constipation in People Receiving Palliative Care (Review)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Laxatives for the management of constipation in people receiving palliative care (Review) Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2015, Issue 5 http://www.thecochranelibrary.com Laxatives for the management of constipation in people receiving palliative care (Review) Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 4 METHODS ...................................... 4 RESULTS....................................... 7 Figure1. ..................................... 8 Figure2. ..................................... 9 Figure3. ..................................... 10 DISCUSSION ..................................... 13 AUTHORS’CONCLUSIONS . 14 ACKNOWLEDGEMENTS . 14 REFERENCES ..................................... 15 CHARACTERISTICSOFSTUDIES . 17 DATAANDANALYSES. 26 ADDITIONALTABLES. 26 APPENDICES ..................................... 28 WHAT’SNEW..................................... 35 HISTORY....................................... 35 CONTRIBUTIONSOFAUTHORS . 36 DECLARATIONSOFINTEREST . 36 SOURCESOFSUPPORT . 36 DIFFERENCES -

Enhancement of Some Mechanical Properties of Polyethylene Glycol by Adding Carboxymethyl Cellulose As a Blends and Applied in Wood Glue
Available online at www.worldscientificnews.com WSN 21 (2015) 12-23 EISSN 2392-2192 Enhancement of Some Mechanical Properties of Polyethylene Glycol by Adding Carboxymethyl Cellulose as a Blends and Applied in Wood Glue Karrar Abd-Ali Obeid1,*, Abdul-Kareem J. Al-Bermany1, Majeed Ali Habeeb2 1Department of Physics, College of Science, University of Babylon, Iraq 2 Department of Physics, College of Education for Pure Science, University of Babylon, Iraq *E-mail address: [email protected] ABSTRACT The (PEG/CMC) blends were prepared by liquids mixing method, the appropriate concentrations of PEG were (0.1-0.2 to 0.8 g/mL)% are dissolved in (500 mL) of distilled water under stirring without heat for (20 min.) then added the CMC with two weights (0.25 and 0.5) g for each PEG concentrates at (288 K). In order to evaluate the mechanical properties of (PEG/CMC) blends the ultrasonic measurements were performed at these samples, these properties are ultrasonic velocity, compressibility, acoustic impedance and bulk modulus, were made at fixed frequency (f = 25 kHz), another acoustic mechanical properties were measured and calculated at a same time such as the ultrasonic wave amplitude absorption by using oscilloscope, then calculated the absorption coefficient and the transmittance ratio of the sound. It was found that there is significant relationship between ultrasonic velocity and material properties results show that adding PEG effects on the density then on the absorption of the ultrasonic waves inside the blend samples. Finally applied this blend in various concentrations as additive to imported wood glue and found that increasing the adhesion force after adding comparing with pure. -

Immediate Hypersensitivity Reactions Caused by Drug Excipients: a Literature Review Caballero ML, Quirce S
REVIEWS Immediate Hypersensitivity Reactions Caused by Drug Excipients: A Literature Review Caballero ML, Quirce S Department of Allergy, La Paz University Hospital, IdiPAZ, Madrid, Spain J Investig Allergol Clin Immunol 2020; Vol. 30(2): 86-100 doi: 10.18176/jiaci.0476 Abstract The European Medicines Agency defines excipients as the constituents of a pharmaceutical form apart from the active substance. Immediate hypersensitivity reactions (IHRs) caused by excipients contained in the formulation of medications have been described. However, there are no data on the prevalence of IHRs due to drug excipients. Clinical manifestations of allergy to excipients can range from skin disorders to life-threatening systemic reactions. The aim of this study was to review the literature on allergy to pharmaceutical excipients and to record the IHRs described with various types of medications, specifically reactions due to the excipients contained in their formulations. The cases reported were sorted alphabetically by type of medication and excipient in order to obtain a list of the excipients most frequently involved for each type of medication. Key words: Allergy. Drug immediate hypersensitivity reaction. Excipient. Pharmaceutical excipients. Resumen La Agencia Europea de Medicamentos define los excipientes como los componentes de una forma farmacéutica diferenciados del principio activo. Se han descrito reacciones de hipersensibilidad inmediata causadas por los excipientes contenidos en la formulación de medicamentos. Sin embargo, no hay datos sobre la prevalencia de dichas reacciones. Las manifestaciones clínicas de la alergia a los excipientes pueden ir desde trastornos de la piel hasta reacciones sistémicas que ponen en peligro la vida. El objetivo de este estudio fue realizar una revisión de la literatura sobre la alergia a los excipientes farmacéuticos y recopilar las reacciones inmediatas descritas con diferentes tipos de medicamento, debido solo a excipientes contenidos en sus formulaciones. -

Prucalopride for the Treatment of Chronic Constipation in Women
Prucalopride for the Treatment of Chronic Constipation in Women: Single Technology Appraisal Response to consultation from Norgine Pharmaceuticals Ltd Comments on clinical assessment 1. The ICER for prucalopride of around £15,000 to £17,000 per QALY gained, whilst probably acceptable for an innovative medicine for a serious or life- threatening condition, is far in excess of what should be considered as acceptable for a laxative. As far as providing value for the NHS is concerned the ICER for Norgine‟s macrogol laxative Movicol is estimated at £250 per QALY gained1, which clearly provides much better value for money. Norgine would therefore question whether prucalopride should be recommended at all by NICE for use in the NHS in England and Wales. 2. If it is considered that prucalopride is cost-effective for use in the NHS in England and Wales, then the preliminary recommendations of the Advisory Committee are not sufficiently precise to avoid doubt as to what the guidance is intended to recommend. For example: (i) It is not clear what is meant by “a clinician with experience of treating chronic constipation.” Many clinicians treat chronic constipation and in numerical terms, nurses probably are involved to a greater extent in managing this condition in primary care than are doctors, and as a result probably have more experience in treating chronic constipation than do primary care physicians. Not all gastroenterologists in secondary care would necessarily qualify as experienced in treating chronic constipation, unless they specialise in the functional bowel disorders. Therefore we would suggest that initially at least the guidance should state that prucalopride therapy should only be initiated by a secondary care physician specialising in the treatment of the functional bowel disorders. -

Comparison of Bran, Ispaghula, and Lactulose on Colon Function in Diverticular Disease
Gut: first published as 10.1136/gut.19.12.1144 on 1 December 1978. Downloaded from Gut, 1978, 19, 1144-1147 Comparison of bran, ispaghula, and lactulose on colon function in diverticular disease M. A. EASTWOOD, A. N. SMITH, W. G. BRYDON, AND J. PRITCHARD From the Wolfson Laboratories, Gastrointestinal Unit, Departments of Medicine and Clinical Surgery, University ofEdinburgh, and Department of Clinical Chemistry, Western General Hospital, Edinburgh SUMMARY Bran, ispaghula (Fybogel), and lactulose were given to three groups of patients with diverticular disease for four weeks. Faecal weights, bile acids, fat and electrolytes, transit time, and colonic motility were estimated before and after treatment. Stool weight increased, notably with Fybogel. Cereal bran had the greatest effect on the transit time, reducing it significantly. There were no changes in faecal bile acids, fat or electrolytes. Coarse bran reduced colonic motility and the number of high pressure waves after food; Fybogel increased the basal pressure and was without effect on the food-stimulated pressures; whereas lactulose influenced neither. All agents paradox- ically equally alleviated symptoms. The symptoms of diverticular disease arise in Each patient was studied at home on his habitual patients who have, in the main, a small stool weight, diet. For one week stool was collected; at the prolonged intestinal transit time, and a raised start of the collection 40 barium impregnated intracolonic pressure (Painter, 1975). The rational markers were taken by mouth (Hinton et al., 1969). basis of the treatment of diverticular disease is Such markers not only give a crude estimate of principally to influence the raised intraluminal transit but act as indicators of the accuracy of http://gut.bmj.com/ pressure. -

United States Patent (19) (11) 4,301,146 Sanvordeker 45) Nov
United States Patent (19) (11) 4,301,146 Sanvordeker 45) Nov. 17, 1981 54 STABILIZATION OF 16-OXYGENATED 56) References Cited PROSTANOIC ACID DERVATIVES U.S. PATENT DOCUMENTS 3,826,823 7/1974. O'Rourke et al. .................... 424/80 75 Inventor: Dilip R. Sanvordeker, Elk Grove 3,965,143 6/1976 Collins et al. ...... 424/305 Village, Ill. 4,058,623 11/1977 Hoffmann et al. ... 424/80 4,127,647 1 1/1978. Sato et al. ............................. 424/78 73 Assignee: G. D. Searle & Co., Skokie, Ill. Primary Examiner-Sam Rosen Attorney, Agent, or Firm-Albert Tockman; W. Dennis Drehkoff Appl. No.: 173,292 21) 57 ABSTRACT A stable solid dosage form of the compound Eme 22 Filed: Jul. 29, 1980 thyl(7-3(a)-hydroxy-2-g-(4(RS)-4-hydroxy-4-methyl trans-1-octen-1-yl)-oxycyclopent-la-ylheptanoate, 51) int. Cl. ................... A61K 31/74; A61K 31/215; said solid dosage form comprising from about 50 to A61K 31/19 about 500 parts of a polymer selected from the group 52 U.S. Cl. ...................................... 424/80; 424/305; consisting of hydroxypropylmethyl cellulose and 424/317; 424/362 polyvinylpyrolidone per part of said compound. 58) Field of Search ................... 424/80, 78, 362, 305, 424/317 22 Claims, No Drawings 4,301,146 1. 2 suitable solvent; (3) adding the drug solution to the STABILIZATION OF 16-OXYGENATED polymer solution; (4) stirring for from about 1 to 5 PROSTANOIC ACID DERVATIVES hours, preferably for about 2 to 4 hours at room temper ature; (5) adding, if desired, up to 1000 parts of a filler U.S. -

Managing Opioid-Induced Constipation
Visual summary MODIFY PAIN RELIEF Managing opioid-induced constipation Is the current dose of opioid needed to control pain? Constipation affects many patients using opioids Could pain be relieved in other ways? to relieve pain associated with advanced cancer Non-opioid analgesics Interventional pain management or terminal disease. Constipation is often multifactorial in such patients, Could an opioid with less constipating effect be used? and opioids may be one of several causes. As well Buprenorphine Transdermal fentanyl as direct treatment of the constipation, adjusting medication regimens and treating exacerbating factors may help to provide relief from bowel transit Is a referral to other specialists needed? symptoms. Palliative care Neuro-gastroenterology Pain clinic ADDRESS EXACERBATING FACTORS DIRECTLY TREAT CONSTIPATION Drugs Non-pharmacological options 5HT3 antagonists Anticholinergics Opioids Increase fluid consumption Increase dietary fibres Antipsychotics Diuretics Iron Increase physical activity (within patients’ capabilities) Calcium supplements Calcium channel blockers Privacy and comfort during defecation Chemotherapies Complementary therapy Thalidomide Vinca alkaloids Positioning on toilet (to relax the puborectalis muscle) knees higher than hips, leaning forward Busulfan Carboplatin with elbows on knees, straightened spine Manual removal Nutritional and if the constipation is severe and refractory to other therapies metabolic issues Pharmacological management Dehydration Reduced food and fluids intake Oral laxatives Poor -

Treatment of Faecal Impaction in Children Using Combined
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Groningen University of Groningen Treatment of fecal impaction in children using combined polyethylene glycol and sodium picosulphate Lamanna, Anthony; Dughetti, Lauren D.; Jordan-Ely, Julie A.; Dobson, Kyla M.; Dynan, Megan; Foo, Adeline; Kooiman, Louise M. P.; Murakami, Naomi; Fiuza, Kaic; Foroughi, Siavash Published in: BJGP open DOI: 10.1002/jgh3.12062 IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2018 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Lamanna, A., Dughetti, L. D., Jordan-Ely, J. A., Dobson, K. M., Dynan, M., Foo, A., ... Southwell, B. R. (2018). Treatment of fecal impaction in children using combined polyethylene glycol and sodium picosulphate. BJGP open, 2(4), 144-151. https://doi.org/10.1002/jgh3.12062 Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. -

Compound Macrogol Oral Powder Sugar Free. Please Tell Your Doctor Or Pharmacist If You Are Taking, Have Recently Taken Or Might Take Any Other Medicines
Package leaflet: Information for the user Compound Macrogol Oral Powder Sugar Free Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. Always take this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse have told you. - Keep this leaflet. You may need to read it again. - Ask your pharmacist if you need more information or advice. - If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4. - You must talk to a doctor if you do not feel better or if you feel worse after 14 days for chronic constipation, or after 3 days for faecal impaction. What is in this leaflet: 1. What Compound Macrogol Oral Powder Sugar Free is and what it is used for 2. What you need to know before you take Compound Macrogol Oral Powder Sugar Free 3. How to take Compound Macrogol Oral Powder Sugar Free 4. Possible side effects 5. How to store Compound Macrogol Oral Powder Sugar Free 6. Contents of the pack and other information 1. What Compound Macrogol Oral Powder Sugar Free is and what it is used for Compound Macrogol Oral Powder Sugar Free contains macrogol 3350 and the electrolytes sodium chloride, sodium hydrogen carbonate and potassium chloride. Macrogol 3350 is a laxative used for the treatment of constipation, especially if you have been constipated for a long time. It is also used to treat a build up of hard faeces in your bowel which may be a result of long-term constipation (this is known as faecal impaction). -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole