Docusate Sodium
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
Final Stakeholder List PDF 190 KB
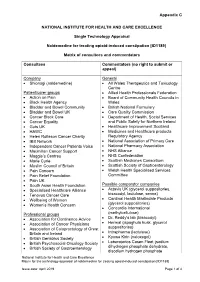
Appendix C NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal Naldemedine for treating opioid-induced constipation [ID1189] Matrix of consultees and commentators Consultees Commentators (no right to submit or appeal) Company General • Shionogi (naldemedine) • All Wales Therapeutics and Toxicology Centre Patient/carer groups • Allied Health Professionals Federation • Action on Pain • Board of Community Health Councils in • Black Health Agency Wales • Bladder and Bowel Community • British National Formulary • Bladder and Bowel UK • Care Quality Commission • Cancer Black Care • Department of Health, Social Services • Cancer Equality and Public Safety for Northern Ireland • Guts UK • Healthcare Improvement Scotland • HAWC • Medicines and Healthcare products • Helen Rollason Cancer Charity Regulatory Agency • IBS Network • National Association of Primary Care • Independent Cancer Patients Voice • National Pharmacy Association • Macmillan Cancer Support • NHS Alliance • Maggie’s Centres • NHS Confederation • Marie Curie • Scottish Medicines Consortium • Muslim Council of Britain • Scottish Society of Gastroenterology • Pain Concern • Welsh Health Specialised Services • Pain Relief Foundation Committee • Pain UK • South Asian Health Foundation Possible comparator companies • Specialised Healthcare Alliance • Actavis UK (glycerol suppositories, • Tenovus Cancer Care bisacodyl, lactulose, senna) • Wellbeing of Women • Cardinal Health Martindale Products • Women’s Health Concern (glycerol suppositories) • Concordia International -

PROVERA Medroxyprogesterone Acetate Tablets 2.5Mg, 5Mg, 10Mg, 100 Mg, 200 Mg Tablets
PROVERA Medroxyprogesterone acetate tablets 2.5mg, 5mg, 10mg, 100 mg, 200 mg tablets What is in this leaflet establish a regular menstrual breast cancer or breast lumps cycle not diagnosed by your doctor This leaflet answers some common bleeding or discharge from questions about PROVERA. It does certain types of cancer including your nipples not contain all the available cancer of the breast, kidney and information. It does not take the endometrium (the lining of the miscarriage place of talking to your doctor or womb). pharmacist. cancer of the womb or ovary PROVERA, in combination with an uncontrolled high blood estrogen containing medicine, is All medicines have risks and pressure. benefits. Your doctor has weighed used to relieve symptoms of the risks of you taking PROVERA menopause in women with an intact Do not take PROVERA if you are against the benefits it is expected to uterus. This is called hormone pregnant or intend to become have for you. replacement therapy (HRT). pregnant. PROVERA is used to protect the If you have any concerns about lining of the uterus while the PROVERA may affect your taking this medicine, ask your estrogens relieve the symptoms of developing baby if you take it doctor or pharmacist. Keep this menopause. PROVERA is not during pregnancy. leaflet with your medicine. suitable as a HRT treatment in women who have undergone a Do not take PROVERA if the You may need to read it again. hysterectomy. packaging is torn or shows signs of tampering. Do not take Your doctor may have prescribed PROVERA after the expiry date What PROVERA is PROVERA for another purpose. -

Liquid Spray Formulations for Buccal Delivery of Cannabinoids
(19) TZZ_¥__T (11) EP 1 361 864 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/08 (2006.01) A61K 9/107 (2006.01) 04.12.2013 Bulletin 2013/49 A61K 36/185 (2006.01) (21) Application number: 02712063.3 (86) International application number: PCT/GB2002/000620 (22) Date of filing: 14.02.2002 (87) International publication number: WO 2002/064109 (22.08.2002 Gazette 2002/34) (54) LIQUID SPRAY FORMULATIONS FOR BUCCAL DELIVERY OF CANNABINOIDS FLÜSSIGE SPRAY FORMULIERUNGEN ZUR BUCCALEN VERABREICHUNG VON CANNABINOIDEN PREPARATIONS DE SPRAY LIQUIDE POUR L’ADMINISTRATION BUCCALE DE CANNABINOIDES (84) Designated Contracting States: (74) Representative: Schiller, Dominic Christopher et AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU al MC NL PT SE TR Harrison Goddard Foote LLP Designated Extension States: 4th Floor, Merchant Exchange RO SI 17-19 Whitworth Street West Manchester M1 5WG (GB) (30) Priority: 14.02.2001 GB 0103638 30.03.2001 US 280044 P (56) References cited: 05.04.2001 US 827158 WO-A-00/25127 WO-A-01/66089 11.05.2001 GB 0111597 WO-A-95/25504 WO-A1-02/32420 07.09.2001 GB 0121715 WO-A1-02/069993 US-A- 3 560 625 12.09.2001 US 951022 • EITAN ALHANATY; ET AL.: "Osmotic fragility of (43) Date of publication of application: liposomes as affected by antihemolytic 19.11.2003 Bulletin 2003/47 compounds" BIOCHIMICA ET BIOPHYSICA ACTA, vol. 339, no. 1, 1974, pages 146-155, (60) Divisional application: XP008008726 10180611.5 / 2 286 793 • PATRICIA S. -

Student Members of the American Chemical Society Poster Presentation Event November 6, 2020 5:30 - 7:00 Pm Stonecipher Lecture Hall
Student Members of the American Chemical Society Poster Presentation Event November 6, 2020 5:30 - 7:00 pm Stonecipher Lecture Hall Author: Brooke Underwood*, Courtney LaPointe, Dr. Jeffrey Boles Contact information: [email protected] Title: Liquid-liquid extraction and ultraviolet visible spectroscopy methods for distinguishing between hemp and marijuana Abstract: In December 2018, cannabis containing less than 0.3% tetrahydrocannabinol (THC), otherwise known as hemp, became legalized due to passage of the Farm Bill (1). This creates problems for law enforcement since current presumptive test kits either 1) don’t work at all or 2) work somewhat in differentiating between legal and illegal hemp crops. This problem exists because most hemp crops and hemp products contain low levels of THC and the carboxylated form, THCA. Our approach involves the advancement of an efficient, mobile, liquid-liquid extraction (LLE) that provides presumptive, qualitative forensic evidence of the chemical extract of a bud or other plant material. This research is focused on developing a kit that functions in a similar manner to NIK kits, commonly used by law enforcement, where all components of the kit are contained within a bag. The current NIK kit for Marijuana provides a false positive when Hemp is placed in the bag, thus creating the need for a more reliable test (2). The evidence would later be sent to a crime lab for definitive analysis and quantitation of THC by ultraviolet-visible spectroscopy (UV-vis). This research has focused on the utilization of liquid-liquid extraction techniques and commercially available stains. The methods presented are rapid (requiring no more than five to six minutes to complete). -

Laxatives for the Management of Constipation in People Receiving Palliative Care (Review)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Laxatives for the management of constipation in people receiving palliative care (Review) Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2015, Issue 5 http://www.thecochranelibrary.com Laxatives for the management of constipation in people receiving palliative care (Review) Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 4 METHODS ...................................... 4 RESULTS....................................... 7 Figure1. ..................................... 8 Figure2. ..................................... 9 Figure3. ..................................... 10 DISCUSSION ..................................... 13 AUTHORS’CONCLUSIONS . 14 ACKNOWLEDGEMENTS . 14 REFERENCES ..................................... 15 CHARACTERISTICSOFSTUDIES . 17 DATAANDANALYSES. 26 ADDITIONALTABLES. 26 APPENDICES ..................................... 28 WHAT’SNEW..................................... 35 HISTORY....................................... 35 CONTRIBUTIONSOFAUTHORS . 36 DECLARATIONSOFINTEREST . 36 SOURCESOFSUPPORT . 36 DIFFERENCES -

Immediate Hypersensitivity Reactions Caused by Drug Excipients: a Literature Review Caballero ML, Quirce S
REVIEWS Immediate Hypersensitivity Reactions Caused by Drug Excipients: A Literature Review Caballero ML, Quirce S Department of Allergy, La Paz University Hospital, IdiPAZ, Madrid, Spain J Investig Allergol Clin Immunol 2020; Vol. 30(2): 86-100 doi: 10.18176/jiaci.0476 Abstract The European Medicines Agency defines excipients as the constituents of a pharmaceutical form apart from the active substance. Immediate hypersensitivity reactions (IHRs) caused by excipients contained in the formulation of medications have been described. However, there are no data on the prevalence of IHRs due to drug excipients. Clinical manifestations of allergy to excipients can range from skin disorders to life-threatening systemic reactions. The aim of this study was to review the literature on allergy to pharmaceutical excipients and to record the IHRs described with various types of medications, specifically reactions due to the excipients contained in their formulations. The cases reported were sorted alphabetically by type of medication and excipient in order to obtain a list of the excipients most frequently involved for each type of medication. Key words: Allergy. Drug immediate hypersensitivity reaction. Excipient. Pharmaceutical excipients. Resumen La Agencia Europea de Medicamentos define los excipientes como los componentes de una forma farmacéutica diferenciados del principio activo. Se han descrito reacciones de hipersensibilidad inmediata causadas por los excipientes contenidos en la formulación de medicamentos. Sin embargo, no hay datos sobre la prevalencia de dichas reacciones. Las manifestaciones clínicas de la alergia a los excipientes pueden ir desde trastornos de la piel hasta reacciones sistémicas que ponen en peligro la vida. El objetivo de este estudio fue realizar una revisión de la literatura sobre la alergia a los excipientes farmacéuticos y recopilar las reacciones inmediatas descritas con diferentes tipos de medicamento, debido solo a excipientes contenidos en sus formulaciones. -

Vaginal Administration of Contraceptives
Scientia Pharmaceutica Review Vaginal Administration of Contraceptives Esmat Jalalvandi 1,*, Hafez Jafari 2 , Christiani A. Amorim 3 , Denise Freitas Siqueira Petri 4 , Lei Nie 5,* and Amin Shavandi 2,* 1 School of Engineering and Physical Sciences, Heriot-Watt University, Edinburgh EH14 4AS, UK 2 BioMatter Unit, École Polytechnique de Bruxelles, Université Libre de Bruxelles, Avenue F.D. Roosevelt, 50-CP 165/61, 1050 Brussels, Belgium; [email protected] 3 Pôle de Recherche en Gynécologie, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, 1200 Brussels, Belgium; [email protected] 4 Fundamental Chemistry Department, Institute of Chemistry, University of São Paulo, Av. Prof. Lineu Prestes 748, São Paulo 05508-000, Brazil; [email protected] 5 College of Life Sciences, Xinyang Normal University, Xinyang 464000, China * Correspondence: [email protected] (E.J.); [email protected] (L.N.); [email protected] (A.S.); Tel.: +32-2-650-3681 (A.S.) Abstract: While contraceptive drugs have enabled many people to decide when they want to have a baby, more than 100 million unintended pregnancies each year in the world may indicate the contraceptive requirement of many people has not been well addressed yet. The vagina is a well- established and practical route for the delivery of various pharmacological molecules, including contraceptives. This review aims to present an overview of different contraceptive methods focusing on the vaginal route of delivery for contraceptives, including current developments, discussing the potentials and limitations of the modern methods, designs, and how well each method performs for delivering the contraceptives and preventing pregnancy. -

Prucalopride for the Treatment of Chronic Constipation in Women
Prucalopride for the Treatment of Chronic Constipation in Women: Single Technology Appraisal Response to consultation from Norgine Pharmaceuticals Ltd Comments on clinical assessment 1. The ICER for prucalopride of around £15,000 to £17,000 per QALY gained, whilst probably acceptable for an innovative medicine for a serious or life- threatening condition, is far in excess of what should be considered as acceptable for a laxative. As far as providing value for the NHS is concerned the ICER for Norgine‟s macrogol laxative Movicol is estimated at £250 per QALY gained1, which clearly provides much better value for money. Norgine would therefore question whether prucalopride should be recommended at all by NICE for use in the NHS in England and Wales. 2. If it is considered that prucalopride is cost-effective for use in the NHS in England and Wales, then the preliminary recommendations of the Advisory Committee are not sufficiently precise to avoid doubt as to what the guidance is intended to recommend. For example: (i) It is not clear what is meant by “a clinician with experience of treating chronic constipation.” Many clinicians treat chronic constipation and in numerical terms, nurses probably are involved to a greater extent in managing this condition in primary care than are doctors, and as a result probably have more experience in treating chronic constipation than do primary care physicians. Not all gastroenterologists in secondary care would necessarily qualify as experienced in treating chronic constipation, unless they specialise in the functional bowel disorders. Therefore we would suggest that initially at least the guidance should state that prucalopride therapy should only be initiated by a secondary care physician specialising in the treatment of the functional bowel disorders. -

And Dietary Measures Literature Review on the Effect of Laxative Treatment
Downloaded from adc.bmj.com on 28 August 2008 Currently recommended treatments of childhood constipation are not evidence based. A systematic literature review on the effect of laxative treatment and dietary measures Maaike A. M. Pijpers, Merit Tabbers, Marc A. Benninga and Marjolein Y. Berger Arch. Dis. Child. published online 19 Aug 2008; doi:10.1136/adc.2007.127233 Updated information and services can be found at: http://adc.bmj.com/cgi/content/abstract/adc.2007.127233v1 These include: Rapid responses You can respond to this article at: http://adc.bmj.com/cgi/eletter-submit/adc.2007.127233v1 Email alerting Receive free email alerts when new articles cite this article - sign up in the box at the service top right corner of the article Notes Online First contains unedited articles in manuscript form that have been peer reviewed and accepted for publication but have not yet appeared in the paper journal (edited, typeset versions may be posted when available prior to final publication). Online First articles are citable and establish publication priority; they are indexed by PubMed from initial publication. Citations to Online First articles must include the digital object identifier (DOIs) and date of initial publication. To order reprints of this article go to: http://journals.bmj.com/cgi/reprintform To subscribe to Archives of Disease in Childhood go to: http://journals.bmj.com/subscriptions/ Downloaded from adc.bmj.com on 28 August 2008 ADC Online First, published on August 19, 2008 as 10.1136/adc.2007.127233 Currently recommended treatments of childhood constipation are not evidence based. A systematic literature review on the effect of laxative treatment and dietary measures. -

Assessment Report on Cassia Senna L
European Medicines Agency Evaluation of Medicines for Human Use London, 27 April 2007 Doc. Ref. EMEA/HMPC/51868/2006 Corr. COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) ASSESSMENT REPORT ON CASSIA SENNA L. AND CASSIA ANGUSTIFOLIA VAHL, FOLIUM Herbal substance Cassia senna L. (C. acutifolia Delile) [Alexandrian or Khartoum senna] or Cassia angustifolia Vahl [Tinnevelly senna], folium (senna leaf) or a mixture of the two species Herbal preparation dried leaflets, standardised; standardised herbal preparations thereof Pharmaceutical forms Herbal substance for oral preparation Rapporteur Dr C. Werner Assessor Dr B. Merz Superseded 7 Westferry Circus, Canary Wharf, London, E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 75 23 70 51 E-mail: [email protected] http://www.emea.europa.eu ©EMEA 2007 Reproduction and/or distribution of this document is authorised for non commercial purposes only provided the EMEA is acknowledged TABLE OF CONTENTS I. Introduction 3 II. Clinical Pharmacology 3 II.1 Pharmacokinetics 3 II.1.1 Phytochemical characterisation 3 II.1.2 Absorption, metabolism and excretion 4 II.1.3 Progress of action 5 II.2 Pharmacodynamics 5 II.2.1 Mode of action 5 • Laxative effect 5 II.2.2 Interactions 7 III. Clinical Efficacy 7 III.1 Dosage 7 III.2 Clinical studies 8 III.2.1 Constipation 8 III.2.2 Irritable bowel syndrome 10 III.2.3 Bowel cleansing 11 III.3 Clinical studies in special populations 15 III.3.1 Use in children 15 III.3.2 Use during pregnancy and lactation 16 III.4 Traditional use 17 IV. -

Protocol for Look-Alike and Sound-Alike Drugs
Community Mental Health for Central Michigan PROTOCOL FOR LOOK-ALIKE AND SOUND-ALIKE DRUGS This protocol should be posted in all licensed residential group homes who contract with Community Mental Health for Central Michigan ADMINISTRATIVE GUIDELINE In an effort to improve medication safety and to meet The Joint Commission’s National Patient Safety Goal Number 3, Community Mental Health for Central Michigan (CMHCM) has identified a process to address sound-alike and look-alike drugs. The Performance Improvement Team that developed this guideline will continue to monitor new drugs on the market as they are released, review the list included with this guideline on an annual basis, and provide appropriate updates. Appropriate action to prevent errors involving the interchange of these drugs will be taken. Each CMHCM site (including direct and provider network sites) where medication is distributed or administered will post the attached lists and implement a plan for preventing drug mix-ups. This plan may consist of but not be limited to: Listing both the brand and generic names on medication records. Storing products with look-alike or sound-alike names in different locations. Employing double checks in the distribution process. Affixing “name alert” stickers to areas where look-alike or sound-alike products are stored. Changing the appearance of look-alike product names on pharmacy labels, computer screens, shelf labels and bins, and medication records by highlighting, through bold face, color, and/or tall man letters, the parts of the names that are different (e.g. hydrOXYzine, hydrALAzine). Having physicians write prescriptions using both the brand and generic names. -

Drugs Used in Treating Constipation and IBS
Drugs used in treating constipation and IBS Define constipation Know the different symptoms of constipation Know the different lines of treatment of constipation Identify the different types of laxatives Discuss the pharmacokinetics, dynamics, side effects and uses of laxatives Discuss the difference between different treatment including bulk forming laxatives, osmotic laxatives, stimulant laxatives And stool softeners (lubricants). Define bowel syndrome (IBS). Identify the pharmacokinetics, dynamics, side effects and uses of drugs used for IBS. extra information and further explanation important doctors notes Drugs names Mnemonics Kindly check the editing file before studying this document constipation and IBS Very useful. Don’t miss it Infrequent defecation, often with straining and the passage of hard, uncomfortable stools. - May be accompanied by other symptoms: Abdominal discomfort and rectal pain, Flatulence, Loss of appetite, Lethargy & Depression. Decreased motility in colon: Decrease in water and fiber Difficulty in evacuation: Drug-induced: contents of diet. 1. Local painful conditions: 1. Anticholinergic agents “Unbalanced diet” anal fissures, piles. 2. Opioids 2. Lack of muscular 3. Iron exercise. 4. Antipsychotics. 5. Anti-depressant, anti- histamine, has anticholinergic effect Adequate fluid intake High fiber contents in diet Use drugs (laxatives or Regulation of bowel habit. purgatives more watery effect) Regular exercise Avoid drugs causing constipation. • Drugs that hasten the transit of food through the gastrointestinal tract are called (Or we say the drugs that increase GI motility) (محركات) or purgatives(ملينات) laxatives • Loosen stools and increase bowel movement Bulk forming laxatives (increase the size of stool ) • Increase volume of non-absorbable solid residue. Osmotic laxatives (cause water withdrawal by sugar or salt) • Increase water content in large intestine.