Laxatives Or Methylnaltrexone for the Management of Constipation in Palliative Care Patients (Review)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemicals Used for Chemical Manufacturing Page 1 of 2
Chemicals used for Chemical Manufacturing Page 1 of 2 Acetic Acid (Glacial, 56%) Glycol Ether PMA Acetone Glycol Ether PNB Acrylic Acid Glycol Ether PNP Activated Carbon Glycol Ether TPM Adipic Acid Glycols Aloe Vera Grease Aluminum Stearate Gum Arabic Aluminum Sulfate Heat Transfer Fluids Amino Acid Heptane Ammonium Acetate Hexane Ammonium Bicarbonate Hydrazine Hydrate Ammonium Bifluoride Hydrochloric Acid (Muriatic) Ammonium Chloride Hydrogen Peroxide Ammonium Citrate Hydroquinone Ammonium Hydroxide Hydroxylamine Sulfate Ammonium Laureth Sulfate Ice Melter Ammonium Lauryl Sulfate Imidazole Ammonium Nitrate Isobutyl Acetate Ammonium Persulfate Isobutyl Alcohol Ammonium Silicofluoride Calcium Stearate Dipropylene Glycol Isopropanolamine Ammonium Sulfate Carboxymethylcellulose Disodium Phosphate Isopropyl Acetate Antifoams Caustic Potash D'Limonene Isopropyl Alcohol Antifreeze Caustic Soda (All Grades) Dodecylbenzene Sulfonic Acid Isopropyl Myristate Antimicrobials Caustic Soda (Beads, Prills) (DDBSA) Isopropyl Palmitate Antimony Oxide Cetyl Alcohol Dowfrost Itaconic Acid Aqua Ammonia Cetyl Palmitate Dowfrost HD Jojoba Oil Ascorbic Acid Chlorine, Granular Dowtherm SR-1 Keratin Barium Carbonate Chloroform Dowtherm 4000 Lactic Acid Barium Chloride Chromic Acid EDTA Lanolin Beeswax Citric Acid (Dry and Liquid) EDTA Plus Lauric Acid Bentonite Coal Epsom Salt Lauryl Alcohol Benzaldehyde Cocamide DEA Ethyl Acetate Lecithin Benzoic Acid Copper Nitrate Ethyl Alcohol (Denatured) Lime Benzyl Alcohol Copper Sulfate Ethylene Glycol Linoleic Acid Bicarbonate -

Potassium-Magnesium Citrate Is an Effective Prophylaxis Against Recurrent Calcium Oxalate Nephrolithiasis
0022-5347/97/1586-2069$03.00/0 JOURNAL OF UROLOGY Vol. 158,2069-2073, December 1997 Copyright Q 1997 by AMERICANUROLOGICAL ASS~CIATION, INC. Printed in U.S.A. POTASSIUM-MAGNESIUM CITRATE IS AN EFFECTIVE PROPHYLAXIS AGAINST RECURRENT CALCIUM OXALATE NEPHROLITHIASIS BRUCE ETTINGER,* CHARLES Y. C. PAK, JOHN T. CITRON, CARL THOMAS, BEVERLEY ADAMS-HIJET AND ARLINE VANGESSEL From the Diuision of Research, Kaiser Permanente Medical Care Program, Oakland, California, the Department of Mineral Metabolism, Center for Mineral Metabolism and Clinical Research, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas, Department of Medicine, Kaiser Permanente Medical Center, Walnut Creek, California, Department of Urology, Kaiser Permanente Medical Center, San Francisco, California, and Kaiser Foundation Research Institute, Kaiser Foundation Hospitals, Oakland, California ABSTRACT Purpose: We examined the efficacy of potassium-magnesium citrate in preventing recurrent calcium oxalate kidney calculi. Materials and Methods: We conducted a prospective double-blind study of 64 patients who were randomly assigned to receive placebo or potassium-magnesium citrate (42 mEq. potassium, 21 mEq. magnesium, and 63 mEq. citrate) daily for up to 3 years. Results. New calculi formed in 63.6%of subjects receiving placebo and in 12.9%of subjects receiving potassium-magnesiumcitrate. When compared with placebo, the relative risk of treat- ment failure for potassium-magnesium citrate was 0.16 (95%confidence interval 0.05 to 0.46). potassium-magnesium citrate had a statistically significant effect (relative risk 0.10,95%confi- dence interval 0.03 to 0.36) even after adjustment for possible confounders, including age, pretreatment calculous event rate and urinary biochemical abnormalities. -

Laxatives for the Management of Constipation in People Receiving Palliative Care (Review)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Laxatives for the management of constipation in people receiving palliative care (Review) Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2015, Issue 5 http://www.thecochranelibrary.com Laxatives for the management of constipation in people receiving palliative care (Review) Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 4 METHODS ...................................... 4 RESULTS....................................... 7 Figure1. ..................................... 8 Figure2. ..................................... 9 Figure3. ..................................... 10 DISCUSSION ..................................... 13 AUTHORS’CONCLUSIONS . 14 ACKNOWLEDGEMENTS . 14 REFERENCES ..................................... 15 CHARACTERISTICSOFSTUDIES . 17 DATAANDANALYSES. 26 ADDITIONALTABLES. 26 APPENDICES ..................................... 28 WHAT’SNEW..................................... 35 HISTORY....................................... 35 CONTRIBUTIONSOFAUTHORS . 36 DECLARATIONSOFINTEREST . 36 SOURCESOFSUPPORT . 36 DIFFERENCES -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -
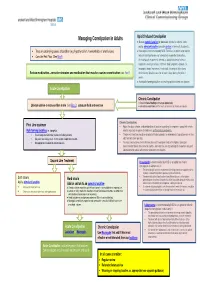
Managing Constipation in Adults
Managing Constipation in Adults Opio id Induced Constipation ● Use an osmotic laxative (or docusate which also softens stools and a stimulant laxative (consider dantron in terminally ill patients). • Treat any underlying causes of condition (e.g. hypothyroidism, haemorrhoids or anal fissures) ● Naloxegol is recommended by NICE TA345 as an option when opioid induced constipation has not adequately responded to laxatives. • Consider Red Flags (See Box A ) An inadequate response is defined as opioid-induced of at least moderate severity in at least 1of the 4 stool symptoms domain (i.e. incomplete bowel movement, hard stool, straining or false alarm) Review medication –consider stopping any medication that may be causing constipation (see Box B ) whilst taking1 laxative class for at least 4 days during the prior 2 weeks. ● Avoid bulk -forming l axatives for treating opioid induced constipation Acute Constipation Chronic Constipation ● Check for faecal loading and manage appropriately Lifestyle advise -to increase fibre in diet (see Box C ), adequate fluid and exercise ● Set realistic expectations for the results of treatment of chronic constipation. Chronic Constipation: First Line treatment • Adjust the dose, choice, and combination of laxative according to symptoms, speed with which Bulk forming laxatives i.e. ispaghula. relief is required, response to treatment, and individual preference. • Ensure adequate fluid intake (caution- frail elderly patients) • The dose of laxative should be gradually titrated upwards (or downwards) to produce one or two • May take several days to act- so not suitable if rapid relief required. soft, formed stools per day. • Not appropriate for opiod induced constipation. • If at least two laxatives (from different classes) have been tried at the highest tolerated recommended doses for at least 6 months, consider the use of prucalopride in women only and lubiprostone for adults with chronic idiopathic constipation. -

COMPASS Therapeutic Notes on the Management of Chronic Constipation in Primary Care
COMPASS Therapeutic Notes on the Management of Chronic Constipation in Primary Care In this issue: Glossary Page Chronic Constipation lasting longer than 3 months Introduction and background 1 constipation Drugs used in the management of Functional Chronic constipation without a known cause. Also known as primary 3 chronic constipation constipation constipation or idiopathic constipation Bulk-forming laxatives 4 Gastrocolic The occurrence of peristalsis following the entrance of food into the Osmotic laxatives 5 response empty stomach Stimulant laxatives 6 IBS Irritable Bowel Syndrome IBS-C Irritable Bowel Syndrome with Constipation Faecal softeners 7 Melanosis Dark brownish black pigmentation of the mucous membrane of the colon Peripheral opioid-receptor 7 coli due to the deposition of pigment in macrophages antagonists Myenteric Part of the enteric nervous system with an important role in regulating 5HT4 – receptor agonists 7 plexus gut motility Managing constipation in 8 NNT Number Needed to Treat pregnancy and breastfeeding OTC Over The Counter RCT Randomised Controlled Trial Secondary Constipation caused by a drug or medical condition. Secondary constipation constipation is also known as organic constipation SmPC Summary of Product Characteristics A twisting or looping of the bowel resulting in obstruction; can be life- Volvulus threatening Successful completion of the assessment questions at the end of this issue will provide you with 2 hours towards your CPD/CME requirements. Further copies of this and any other edition in the -

3.2.2 Misuse of Stimulant Laxatives
Medicines Adverse Reactions Committee Meeting date 10/06/2021 Agenda item 3.2.2 Title Misuse of stimulant laxatives Submitted by Medsafe Pharmacovigilance Paper type For advice Team Active ingredient Product name Sponsor Bisacodyl Bisacodyl Laxative (Pharmacy Health) PSM Healthcare Limited trading as API tablet Consumer Brands Dulcolax tablet Sanofi-Aventis New Zealand Limited Dulcolax Suppository Sanofi-Aventis New Zealand Limited *Lax-Suppositories Bisacodyl AFT Pharmaceuticals Limited *Lax-Tab tablet AFT Pharmaceuticals Limited Docusate sodium *Coloxyl tablet Pharmacy Retailing (New Zealand) Limited trading as Healthcare Logistics Docusate sodium + Coloxyl with Senna tablet Pharmacy Retailing (New Zealand) sennosides Limited trading as Healthcare Logistics *Laxsol tablet Pharmacy Retailing (New Zealand) Limited trading as Healthcare Logistics Glycerol *Glycerol Suppositories PSM Healthcare Limited trading as API Consumer Brands Sennosides *Senokot tablet Reckitt Benckiser (New Zealand) Limited Sodium picosulfate Dulcolax SP Drops oral solution Sanofi-Aventis New Zealand Limited PHARMAC funding *Pharmaceutical Schedule Lax-Tab tablets, Lax-Suppositories Bisacodyl, Coloxyl tablets and Glycerol Suppositories are fully-funded only on a prescription. Senokot tablets are part- funded. Previous MARC Misuse of stimulant laxatives has not been discussed previously. meetings International action Following a national safety review published in August 2020, the MHRA in the UK has introduced pack size restrictions, revised recommended ages for use -

1: Gastro-Intestinal System
1 1: GASTRO-INTESTINAL SYSTEM Antacids .......................................................... 1 Stimulant laxatives ...................................46 Compound alginate products .................. 3 Docuate sodium .......................................49 Simeticone ................................................... 4 Lactulose ....................................................50 Antimuscarinics .......................................... 5 Macrogols (polyethylene glycols) ..........51 Glycopyrronium .......................................13 Magnesium salts ........................................53 Hyoscine butylbromide ...........................16 Rectal products for constipation ..........55 Hyoscine hydrobromide .........................19 Products for haemorrhoids .................56 Propantheline ............................................21 Pancreatin ...................................................58 Orphenadrine ...........................................23 Prokinetics ..................................................24 Quick Clinical Guides: H2-receptor antagonists .......................27 Death rattle (noisy rattling breathing) 12 Proton pump inhibitors ........................30 Opioid-induced constipation .................42 Loperamide ................................................35 Bowel management in paraplegia Laxatives ......................................................38 and tetraplegia .....................................44 Ispaghula (Psyllium husk) ........................45 ANTACIDS Indications: -

Cycle Stability and Hydration Behavior of Magnesium Oxide and Its Dependence on the Precursor-Related Particle Morphology
nanomaterials Article Cycle Stability and Hydration Behavior of Magnesium Oxide and Its Dependence on the Precursor-Related Particle Morphology Georg Gravogl 1,2, Christian Knoll 2,3 , Jan M. Welch 4, Werner Artner 5, Norbert Freiberger 6, Roland Nilica 6, Elisabeth Eitenberger 7, Gernot Friedbacher 7, Michael Harasek 3 , Andreas Werner 8, Klaudia Hradil 5, Herwig Peterlik 9, Peter Weinberger 2 , Danny Müller 2,* and Ronald Miletich 1 1 Department of Mineralogy and Crystallography, University of Vienna, Althanstraße 14, 1090 Vienna, Austria; [email protected] (G.G.); [email protected] (R.M.) 2 Institute of Applied Synthetic Chemistry, TU Wien, Getreidemarkt 9, 1060 Vienna, Austria; [email protected] (C.K.); [email protected] (P.W.) 3 Institute of Chemical, Environmental & Biological Engineering, TU Wien, Getreidemarkt 9, 1060 Vienna, Austria; [email protected] 4 Atominstitut, TU Wien, Stadionallee 2, 1020 Vienna, Austria; [email protected] 5 X-ray Center, TU Wien, Getreidemarkt 9, 1060 Vienna, Austria; [email protected] (W.A.); [email protected] (K.H.) 6 RHI-AG, Magnesitstraße 2, 8700 Leoben, Austria; [email protected] (N.F.); [email protected] (R.N.) 7 Institute of Chemical Technologies and Analytics, TU Wien, Getreidemarkt 9, 1060 Vienna, Austria; [email protected] (E.E); [email protected] (G.F.) 8 Institute for Energy Systems and Thermodynamics, TU Wien, Getreidemarkt 9, 1060 Vienna, Austria; [email protected] 9 Faculty of Physics, University of Vienna, Boltzmanngasse 5, 1090 Vienna, Austria; [email protected] * Correspondence: [email protected]; Tel.: +43-1-5880-1163-740 Received: 31 August 2018; Accepted: 2 October 2018; Published: 7 October 2018 Abstract: Thermochemical energy storage is considered as an auspicious method for the recycling of medium-temperature waste heat. -

Impact of a Drugs & Therapeutics Backgrounder on Docusate Utilization
Impact of a Drugs & Therapeutics Backgrounder on Docusate Utilization Darren Pasay, B.Sc.Pharm Drug Stewardship Pharmacist (Central Zone) [email protected] Disclosure I have no actual or potential conflict of interest in relation to this topic or presentation. Drug Stewardship in Alberta Health Services • Drug Stewardship (DS) Team established in 2012 • “The shared responsibility of Drugs & Safety Therapeutics Committee (DTC), prescribers, pharmacy and care units to ensure Sustainability medications are used in a manner that maximizes the effectiveness, safety and sustainability of care for our patients” Effectiveness • Research and project support Drugs & Therapeutics Backgrounders (DTBs) • Frontline staff wanted background and support on DTC/formulary issues1 • One page document, meant to enhance conversations with prescribers • Supported by content experts, based upon DTC directions • Published 6 times/year • Followed by 2 interactive webinars for each edition 1. Pasay Darren K, Chow Sheldon JS, Bresee Lauren C, Guirguis Micheal, Slobodan Jeremy. Assessment of current antimicrobial stewardship policies and resources: a focus group project. Healthcare Infection 2015; 20: 7–15. Docusate • Over-the-counter stool softener • It is clear, based on published peer-reviewed literature, that docusate is ineffective for the prevention or treatment of constipation • Open listed on AHS Provincial Drug Formulary • Limited coverage on Alberta Drug Benefit List (palliative care only) Docusate DTB • Preliminary work o ~ 2 million doses/year dispensed • Influences o Some LTC sites have eliminated o Engrained in practice use already • Part of pharmacy, medicine, nursing curriculums • Order sets, Pre-printed care • What are the costs of? orders (PPCO) o Procurement o Seen as innocuous, safe o Processing, dispensing o Medication administration o Medication burden BOTTOM LINE: Docusate is no more effective than placebo for the prevention and treatment of constipation. -

And Dietary Measures Literature Review on the Effect of Laxative Treatment
Downloaded from adc.bmj.com on 28 August 2008 Currently recommended treatments of childhood constipation are not evidence based. A systematic literature review on the effect of laxative treatment and dietary measures Maaike A. M. Pijpers, Merit Tabbers, Marc A. Benninga and Marjolein Y. Berger Arch. Dis. Child. published online 19 Aug 2008; doi:10.1136/adc.2007.127233 Updated information and services can be found at: http://adc.bmj.com/cgi/content/abstract/adc.2007.127233v1 These include: Rapid responses You can respond to this article at: http://adc.bmj.com/cgi/eletter-submit/adc.2007.127233v1 Email alerting Receive free email alerts when new articles cite this article - sign up in the box at the service top right corner of the article Notes Online First contains unedited articles in manuscript form that have been peer reviewed and accepted for publication but have not yet appeared in the paper journal (edited, typeset versions may be posted when available prior to final publication). Online First articles are citable and establish publication priority; they are indexed by PubMed from initial publication. Citations to Online First articles must include the digital object identifier (DOIs) and date of initial publication. To order reprints of this article go to: http://journals.bmj.com/cgi/reprintform To subscribe to Archives of Disease in Childhood go to: http://journals.bmj.com/subscriptions/ Downloaded from adc.bmj.com on 28 August 2008 ADC Online First, published on August 19, 2008 as 10.1136/adc.2007.127233 Currently recommended treatments of childhood constipation are not evidence based. A systematic literature review on the effect of laxative treatment and dietary measures. -

National Institute for Clinical Excellence
Appendix C NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal (STA) Lubiprostone for treating opioid-induced constipation in people with chronic, non-cancer pain Matrix of consultees and commentators Consultees Commentators (no right to submit or appeal) Manufacturers/sponsors General Sucampo Pharma Europe Allied Health Professionals Federation (lubiprostone) Board of Community Health Councils in Wales Patient/carer groups British National Formulary Action on Pain Care Quality Commission Afiya Trust Commissioning Support Appraisals Black Health Agency Service Bladder and Bowel Foundation Department of Health, Social Services Equalities National Council and Public Safety for Northern Ireland Muslim Council of Britain Healthcare Improvement Scotland Muslim Health Network Medicines and Healthcare products Pain Concern Regulatory Agency Pain Relief Foundation National Association of Primary Care Pain UK National Pharmacy Association PromoCon NHS Alliance South Asian Health Foundation NHS Commercial Medicines Unit Specialised Healthcare Alliance NHS Confederation IBS Network Public Health Wales NHS Trust Scottish Medicines Consortium Professional groups Association of Coloproctology of Great Comparator manufacturers Britain and Ireland Abbott Laboratories UK (lactulose) Association for Continence Advice Actavis UK (glycerol suppositories), Association for Palliative Medicine Amdipharm (methylcellulose) British Geriatrics Society Bell Sons & Co (Druggists) Limited British Pain