Solubility of Ethylene in Methyl Propionate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pdf 462.69 K
Journal of Oil, Gas and Petrochemical Technology Vol. 5, No. 1 , pp. 63 - 75 6 3 The Investigation of Purity Improvement for the Production of Methyl Propionate in Different Types of Batch Distillation Systems Dhia Y. Aqar * 1 , Hassan H. Al Alak 2 , N. Rahmanian 3 , I.M. Mujtaba 3 1 Ministry of Oil, South Refineries Company, Basra, Iraq 2 Department of Materials Engineering, Faculty of Engineering, University of Kufa, Najaf, Iraq 3 Faculty of Engineering and Informatics, University of Bradford, Bradford, UK ARTICLE INFO ABSTRACT Received: January 06, 2018 Methyl propionate, also known as methyl propanoate, Accepted: September 08, 2018 is a clear colourless liquid with a characteristic odour (fruity smel l and taste). In this study , the formation of methyl propionate through the esterification o Keywords : propionic acid and methanol was inves tigated in the Methyl propionate reactive distillation system using a conventional (CBD), CBD single feed (SF - SBD), and double feed semi - batch SF - SBD distillatio n (DF - SBD) columns for the first time . The Double feed semi - batch distillation performances measure o f these distillation systems (DF - SBD) were evaluated in terms of min imum batch time for a given separation task. The optimization results clearly * Corresponding author: show ed that the DF - SBD system is a more attractive E - mail: : [email protected] operation in terms of reaction conversion, and maximum achievable purity as compared to the SF SBD, and CBD processes. 64 Dhia Y. Aqar et al. 1. Introduction Methyl propionate is a very important component which has useful applications in a variety of areas in the chemical industry such as solvents for cellulose nitrate, lacquers, plasticizers, chemical intermediates, fragrances, flavors, a raw material in organic synthesis for the production of varnishes, paints, and other chemical compound s such as methyl methacrylate [1] . -

Experimental and Modeling Study of the Thermal Decomposition of Methyl Decanoate Olivier Herbinet, Pierre Alexandre Glaude, Valérie Warth, Frédérique Battin-Leclerc
Experimental and modeling study of the thermal decomposition of methyl decanoate Olivier Herbinet, Pierre Alexandre Glaude, Valérie Warth, Frédérique Battin-Leclerc To cite this version: Olivier Herbinet, Pierre Alexandre Glaude, Valérie Warth, Frédérique Battin-Leclerc. Experimental and modeling study of the thermal decomposition of methyl decanoate. Combustion and Flame, Elsevier, 2011, 158 (7), pp.1288-1300. 10.1016/j.combustflame.2010.11.009. hal-00724939 HAL Id: hal-00724939 https://hal.archives-ouvertes.fr/hal-00724939 Submitted on 23 Aug 2012 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Experimental and modeling study of the thermal decomposition of methyl decanoate Olivier Herbineta,*, Pierre-Alexandre Glaudea, Valérie Wartha and Frédérique Battin-Leclerca a Laboratoire Réactions et Génie des Procédés, Nancy Université, CNRS UPR 3349, BP 20451, 1 rue Grandville, 54001 Nancy, France Abstract The experimental study of the thermal decomposition of methyl decanoate was performed in a jet-stirred reactor at temperatures ranging from 773 to 1123 K, at residence times between 1 and 4 s, at a pressure of 800 Torr (106.6 kPa) and at high dilution in helium (fuel inlet mole fraction of 0.0218). -

Food and Drug Administration, HHS § 172.515
Food and Drug Administration, HHS § 172.515 Common name Scientific name Limitations Tolu ................................................................ Myroxylon balsamum (L.) Harms. Turpentine ...................................................... Pinus palustris Mill. and other Pinus spp. which yield terpene oils exclusively. Valerian rhizome and roots ............................ Valeriana officinalis L. Veronica ......................................................... Veronica officinalis L .................................................. Do. Vervain, European ......................................... Verbena officinalis L ................................................... Do. Vetiver ............................................................ Vetiveria zizanioides Stapf ......................................... Do. Violet, Swiss ................................................... Viola calcarata L. Walnut husks (hulls), leaves, and green nuts Juglans nigra L. or J. regia L. Woodruff, sweet ............................................. Asperula odorata L ..................................................... In alcoholic beverages only Yarrow ............................................................ Achillea millefolium L .................................................. In beverages only; fin- ished beverage thujone free1 Yerba santa .................................................... Eriodictyon californicum (Hook, et Arn.) Torr. Yucca, Joshua-tree ........................................ Yucca brevifolia Engelm. Yucca, -

Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC
Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC R E S E A R C H A N D D E V E L O P M E N T EPA/600/R-06/105 September 2006 Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC By S. H. Hilal Ecosystems Research Division National Exposure Research Laboratory Athens, Georgia U.S. Environmental Protection Agency Office of Research and Development Washington, DC 20460 NOTICE The information in this document has been funded by the United States Environmental Protection Agency. It has been subjected to the Agency's peer and administrative review, and has been approved for publication. Mention of trade names of commercial products does not constitute endorsement or recommendation for use. ii ABSTRACT SPARC (SPARC Performs Automated Reasoning in Chemistry) chemical reactivity models were extended to calculate hydrolysis rate constants for carboxylic acid ester and phosphate ester compounds in aqueous non- aqueous and systems strictly from molecular structure. The energy differences between the initial state and the transition state for a molecule of interest are factored into internal and external mechanistic perturbation components. The internal perturbations quantify the interactions of the appended perturber (P) with the reaction center (C). These internal perturbations are factored into SPARC’s mechanistic components of electrostatic and resonance effects. External perturbations quantify the solute-solvent interactions (solvation energy) and are factored into H-bonding, field stabilization and steric effects. These models have been tested using 1471 reliable measured base, acid and general base-catalyzed carboxylic acid ester hydrolysis rate constants in water and in mixed solvent systems at different temperatures. -

Comparative Study of Volatile Compounds in the Fruit of Two Banana Cultivars at Different Ripening Stages
molecules Article Comparative Study of Volatile Compounds in the Fruit of Two Banana Cultivars at Different Ripening Stages Xiaoyang Zhu † , Qiumian Li †, Jun Li, Jun Luo, Weixin Chen and Xueping Li * State Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources/Guangdong Provincial Key Laboratory of Postharvest Science of Fruits and Vegetables, College of Horticulture, South China Agricultural University, Guangzhou 510642, China; [email protected] (X.Z.); [email protected] (Q.L.); [email protected] (J.L.); [email protected] (J.L.); [email protected] (W.C.) * Correspondence: [email protected]; Tel.: +86-20-3829-4892; Fax: +86-20-8528-8280 † These authors contributed equally to this work. Received: 5 September 2018; Accepted: 23 September 2018; Published: 25 September 2018 Abstract: Aromatic compounds are important for fruit quality and can vary among fruit cultivars. Volatile compounds formed during the ripening of two banana cultivars, Brazilian and Fenjiao, were determined using headspace solid-phase micro-extraction (SPME) and gas chromatography coupled with mass spectrometry (GC-MS). These two cultivars exhibited different physiological characteristics during storage. Fenjiao fruit exhibited faster yellowing and softening, a higher respiration rate and greater ethylene production. Also, the soluble sugar content in Fenjiao fruit was much higher than in Brazilian fruit. In total, 62 and 59 volatile compounds were detected in Fenjiao and Brazilian fruits, respectively. The predominant volatile components isoamyl acetate, butanoic acid, 3-methyl-3-methylbutyl ester, hexanal, trans-2-hexenal and 1-hexanol varied during ripening stages. Moreover, esters were more abundant in Fenjiao, and propanoic acid 2-methylbutyl ester, and octanoic acid were only detected in Fenjiao. -

Distributed Representation of Chemical Features and Tunotopic Organization of Glomeruli in the Mouse Olfactory Bulb
Distributed representation of chemical features and tunotopic organization of glomeruli in the mouse olfactory bulb Limei Maa,1, Qiang Qiua, Stephen Gradwohla,2, Aaron Scotta,3, Elden Q. Yua, Richard Alexandera, Winfried Wiegraebea, and C. Ron Yua,b,1 aStowers Institute for Medical Research, Kansas City, MO 64110; and bDepartment of Anatomy and Cell Biology, University of Kansas Medical Center, Kansas City, KS 66160 Edited by John R. Carlson, Yale University, New Haven, CT, and accepted by the Editorial Board February 14, 2012 (received for review October 24, 2011) In the mammalian brain, similar features of the sensory stimuli are features, but recent experiments have provided little support of this often represented in proximity in the sensory areas. However, how notion (7, 23, 24). These studies have suggested that chemotopy at chemical features are represented in the olfactory bulb has been the fine scale does not exist and that the olfactory system violates controversial. Questions have been raised as to whether specific the organization principle found in other sensory systems (7, 25, chemical features of the odor molecules are represented by spatially 26). Moreover, the topographic organization in the bulb is dis- clustered olfactory glomeruli. Using a sensitive probe, we have persed when the projection reaches the piriform cortex (PirC). analyzed the glomerular response to large numbers of odorants at Whereas the mitral cell in the bulb receives input from a single single glomerulus resolution. Contrary to the general view, we find glomerulus, its projection into the PirC extends into a wide area, and a confined cortical region receives input from mitral cells across that the representation of chemical features is spatially distributed in – the olfactory bulb with no discernible chemotopy. -

Process for the Production of Propionate Esters Herstellungsverfahren Von Propionsaureestern Procede De Production D'esters De I'acide Propionique
Europaisches Patentamt (19) European Patent Office Office europeenopeen des brevets EP 0321 054 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) intci.6: C07C 69/24, C07C 67/38, of the grant of the patent: C07C 67/03 06.03.1996 Bulletin 1996/10 (21) Application number: 88202870.7 (22) Date of filing: 13.12.1988 (54) Process for the production of propionate esters Herstellungsverfahren von Propionsaureestern Procede de production d'esters de I'acide propionique (84) Designated Contracting States: (72) Inventors: BE DE FR GB IT NL • Petrus, Leonardus NL-1031 CM Amsterdam (NL) (30) Priority: 15.12.1987 GB 8729267 • De Bruijn, Wouter NL-2596 HR The Hague (NL) (43) Date of publication of application: 21.06.1989 Bulletin 1989/25 (56) References cited: EP-A- 0 01 7 441 EP-A- 0 235 864 (73) Proprietor: SHELL INTERNATIONALE US-A- 3 098 093 RESEARCH MAATSCHAPPIJ B.V. NL-2596 HR Den Haag (NL) Remarks: The file contains technical information submitted after the application was filed and not included in this specification DO ^> o CM CO Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give o notice to the European Patent Office of opposition to the European patent granted. Notice of opposition shall be filed in a written reasoned statement. It shall not be deemed to have been filed until the opposition fee has been paid. (Art. a. 99(1) European Patent Convention). LU Printed by Jouve, 75001 PARIS (FR) EP 0 321 054 B1 Description The invention relates to a process for the production of propionate esters. -

VAPOR-LIQUID EQUILIBRIUM DATA COLLECTION Chemistry Data Series
J. Gmehling U. Onken VAPOR-LIQUID EQUILIBRIUM DATA COLLECTION Esters Supplement 2 Chemistry Data Series Vol. I, Part 5b Published by DECHEMA Gesellschaft fur Chemische Technik und Biotechnologie e. V. Executive Editor: Gerhard Kreysa Bibliographic information published by Die Deutsche Bibliothek Die Deutsche Bibliothek lists this publication in the Deutsche Nationalbibliographie; de tailed bibliographic data is available on the Internet at http://dnb.ddb.de ISBN: 3-89746-041-6 © DECH EMA Gesellschaft fOr Chemische Te chnik und Biotechnologie e. V. Postfach 15 01 04, D-60061 Frankfurt am Main, Germany, 2002 Dieses Werk ist urheberrechtlich geschutzt. Alle Rechte, auch die der Obersetzung, des Nachdrucks und der Vervielfi:Htigung des Buches oder Teilen daraus sind vorbehalten. Kein Teil des Werkes darf ohne schriftliche Genehmigung der DECHEMA in irgendeiner Form (Fotokopie, Mikrofilm oder einem anderen Verfahren), auch nicht fOr Zwecke der Un terrichtsgestaltung, reproduziert oder unter Verwendung elektronischer Systeme verarbei tet, vervielfaltigt oder verbreitet werden. Die Herausgeber Obernehmen fOr die Richtigkeit und Vollstandigkeit der publizierten Daten keinerlei Gewahrleistung. This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, including those of translation, reprinting, reproduction by photo copying machine or similar means. No partof this work may be reproduced, processed or distributed in any form, not even for teaching purposes - by photocopying, microfilm or other processes, or implemented in electronic information storage and retrieval systems - without the written permission of the publishers. The publishers accept no liability for the accuracy and completeness of the published data. This volume of the Chemistry Data Series was printed using acid-free paper. -

Abrasion of Particles 466 Acetamide 185 Acetic Acid
j591 Index a – production, by fermentation (See also abrasion of particles 466 fermentation) acetamide 185 – secondary alcohols resolved using acetic acid 375 lipases 169 Acetobacter 359, 375 – as solvent 83 acetogenic bacteria 384 – synthesis of 144, 145 acetone cyanohydrin 193 – tertiary alcohols 169, 170 N-acetyl L-tyrosine 199 alcohol dehydrogenase (ADH) 19, 39, 71, N-acetyl L-valine 199 143–146, 154, 171, 300, 486 Acinetobacter calcoaceticus 154 alcoholic fermentation. See fermentation acrylamide 142, 314, 347 alcohol oxidase (AOX) 221 – commercial production 184 aldolases 39, 193–197 activation energy 53, 54, 66, 71, 284 – biocatalytic route 541 activator 34, 37, 68, 73, 74, 259 – classes 193, 194 active site of enzyme 42 – retro-aldol reaction 131, 197 activity-based approach, limitations 113 – type I and type II, mechanism of 194 N-acylamino acid racemases 198, 199 – in vivo aldol reaction catalyzed by NeuAc N-acylamino acids 199 aldolase 197 acylases 13, 146, 176, 177, 277, 314, 347 alginate 369, 370, 372 acylation 42, 48, 49, 53, 54, 166, 167, alginic acid 370 170 3-alkyl-substituted 2-cyclohexenones 159 adherent mammalian cells, for amidases 40, 58, 146, 176, 177, 238, 240, biopharmaceuticals production 383, 384 258, 287, 529, 530 adhesion 361, 362, 376, 377, 378, 393, 466 amide bonds 176 adipoyl-7-ADCA 528 – synthesis using proteases and adipoyl-6-APA 527 amidases 176 adsorption, 378. See also enzyme adsorption amino acid dehydrogenases (AADHs) – microorganisms 376 146, 147 aerosols 21, 22 – catalytic mechanism 146 agarose -

Safety Data Sheet
SAFETY DATA SHEET Revision Date 26-Jan-2021 Revision Number 2 SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier Product Description: Methyl propionate Cat No. : A14944 Synonyms Propanoic acid methyl ester CAS-No 554-12-1 Molecular Formula C4 H8 O2 Reach Registration Number - 1.2. Relevant identified uses of the substance or mixture and uses advised against Recommended Use Laboratory chemicals. Uses advised against No Information available 1.3. Details of the supplier of the safety data sheet Company Thermo Fisher (Kandel) GmbH . Erlenbachweg 2 76870 Kandel Germany Tel: +49 (0) 721 84007 280 Fax: +49 (0) 721 84007 300 E-mail address [email protected] www.alfa.com Product safety Tel + +049 (0) 7275 988687-0 1.4. Emergency telephone number Carechem 24: +44 (0) 1235 239 670 (Multi-language emergency number) Poison Information Center Mainz www.giftinfo.uni-mainz.de Telephone: +49(0)6131/19240 Exclusively for customers in Austria: Poison Information Center (VIZ) Emergency call 0-24 clock: +43 1 406 43 43 Office hours: Monday to Friday, 8am to 4pm, tel: +43 1 406 68 98 SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the substance or mixture CLP Classification - Regulation (EC) No 1272/2008 Physical hazards Flammable liquids Category 2 (H225) ______________________________________________________________________________________________ ALFAAA14944 Page 1 / 10 SAFETY DATA SHEET Methyl propionate Revision Date 26-Jan-2021 ______________________________________________________________________________________________ Health hazards Acute Inhalation Toxicity - Vapors Category 4 (H332) Environmental hazards Based on available data, the classification criteria are not met Full text of Hazard Statements: see section 16 2.2. -

(19) United States (12) Patent Application Publication (10) Pub
US 20070088508A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0088508 A1 Childs (43) Pub. Date: Apr. 19, 2007 (54) COCRYSTALLIZATION METHODS Related US. Application Data (60) Provisional application No. 60/721,115, ?led on Sep. (76) Inventor: Scott Childs, Atlanta, GA (US) 28, 2005. Publication Classi?cation Correspondence Address: (51) Int. Cl. FINNEGAN, HENDERSON, FARABOW, GARRETT & DUNNER G06F 19/00 (2006.01) (52) US. Cl. .............................................................. .. 702/19 LLP 901 NEW YORK AVENUE, NW (57) ABSTRACT WASHINGTON, DC 20001-4413 (US) Methods for preparing cocrystals Wherein solutions of active agents in suitable liquids are combined With solutions of (21) Appl. No.: 11/527,395 guests, both With and Without suitable liquids, in an interface region are described herein. Methods for analyzing the (22) Filed: Sep. 27, 2006 cocrystals are also described. Patent Application Publication Apr. 19, 2007 Sheet 1 0f 2 US 2007/0088508 A1 Patent Application Publication Apr. 19, 2007 Sheet 2 0f 2 US 2007/0088508 A1 US 2007/0088508 A1 Apr. 19, 2007 COCRYSTALLIZATION METHODS costs, and manufacturing method may be modi?ed by using [0001] This application claims the bene?t of priority to a cocrystal rather than the active agent alone, or as a salt. provisional application No. 60/721,115, ?led on Sep. 28, [0006] An active agent can be screened for possible coc 2005, the contents of Which are incorporated by reference rystals Where polymorphic forms, hydrates, or solvates are herein. especially problematic. For example, a neutral compound that can only be isolated as amorphous material could be [0002] Cocrystals are crystals that contain tWo or more cocrystalliZed. -
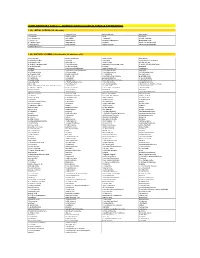
Supplementary Table 1 - Odorant Panels Used in Various Experiments
SUPPLEMENTARY TABLE 1 - ODORANT PANELS USED IN VARIOUS EXPERIMENTS 1 (A). INITIAL SCREEN (25 odorants) (-)-carvone 2-pentyl furan benzaldehyde isoeugenol 1,4-cineole 4-allyl anisole citral limonene 1-methyl pyrrole allyl sulfide eucalyptol methyl salicylate 1-octen-3-ol alpha-ionone gamma-heptalactone propionaldehyde 2,3-butanedione alpha-pinene guaiacol trans-3-hexenoic acid 2-ethyl phenol amyl acetate isobutyric acid trans-cinnamaldehyde 2-methyl pyrazine 1 (B). MIXTURE SCREEN (348 odorants, 58 mixtures of 6) hexyl acetate propyl propionate ethyl caprate isoeugenol 6-methylquinoline geraniol 1-nonanol tetrahydrofurfuryl alcohol isobutyraldehyde alpha-pinene ethyl acrylate phenyl actetate 2-methyl-1-propane thiol 1-methyl pyrrole alpha amyl cinnamaldehyde 3-acetyl-2,5-dimethyl furan 4-methyl anisole methyl myristate decanol bacdanol nonanal 2-methyl butyraldehyde trans-3-hexen-1-ol floralozone pyridine 2-tert-butyl cyclohexanone 1,4-butane dithiol veratrole ethyl heptanoate (+)-rose oxide 2-methyl pyrazine (+/-)-4-heptanolide pelargonic acid heptyl isobutyrate (-)-2-butanol acetophenone cis-3-hexen-1-ol ethyl acetate 3-methylcyclopentanone p-anisaldehyde 3-heptanone eucalyptol pentyl propionate 2-acetyl thiazole isoamyl amine 3-decanone bis-(methylthio) methane 2-phenyl ethanol valeraldehyde 2,5-dimethyl pyrazine 2-methyl anisole styralyl propionate phenetole (+/-)-citronellal 2-methoxy pyrazine ethyl ortho methoxy benzoate alpha-amyl cinnamaldehyde dimethyl acetal 2-nonanone acetaldehyde 2-propenyl phenol 1,3-butane dithiol diethyl sebacate