An Overview on Philippine Estuarine Oomycetes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Collection and Evaluation of Under-Utilized Tropical and Subtropical Fruit Tree Genetic Resources in Malaysia
J]RCAS International Symposium Series No. 3: 27-38 Session 1-3 27 Collection and Evaluation of Under-Utilized Tropical and Subtropical Fruit Tree Genetic Resources in Malaysia WONG, Kai Choo' Abstract Fruit tree genetic resources in Malaysia consist of cultivated and wild species. The cul tivated fruit trees number more than 100 species of both indigenous and introduced species. Among these fruits, some are popular and are widely cultivated throughout the country while others are less known and grown in small localized areas. The latter are the under-utilized fruit species. Apart from these cultivated fruits, there is also in the Malaysian natural forest a diversity of wild fruit tree species which produce edible fruits but are relatively unknown and unutilized. Many of the under-utilized and unutilized fruit species are known to show economic potential. Collection and evaluation of some of these fruit tree genetic resources have been carried out. These materials are assessed for their potential as new fruit trees, as sources of rootstocks for grafting and also as sources of germplasm for breeding to improve the present cultivated fruit species. Some of these potential fruit tree species within the gen era Artocarpus, Baccaurea, Canarium, Dimocarpus, Dialium, Durio, Garcinia, Litsea, Mangif era, Nephelium, Sa/acca, and Syzygium are highlighted. Introduction Malaysian fruit tree genetic resources comprise both cultivated and wild species. There are more than 100 cultivated fruit species of both major and minor fruit crops. Each category includes indigenous as well as introduced species. The major cultivated fruit crops are well known and are commonly grown throughout the country. -

Flora and Fauna of Phong Nha-Ke Bang and Hin Namno, a Compilation Page 2 of 151
Flora and fauna of Phong Nha-Ke Bang and Hin Namno A compilation ii Marianne Meijboom and Ho Thi Ngoc Lanh November 2002 WWF LINC Project: Linking Hin Namno and Phong Nha-Ke Bang through parallel conservation Flora and fauna of Phong Nha-Ke Bang and Hin Namno, a compilation Page 2 of 151 Acknowledgements This report was prepared by the WWF ‘Linking Hin Namno and Phong Nha through parallel conservation’ (LINC) project with financial support from WWF UK and the Department for International Development UK (DfID). The report is a compilation of the available data on the flora and fauna of Phong Nha-Ke Bang and Hin Namno areas, both inside and outside the protected area boundaries. We would like to thank the Management Board of Phong Nha-Ke Bang National Park, especially Mr. Nguyen Tan Hiep, Mr. Luu Minh Thanh, Mr. Cao Xuan Chinh and Mr. Dinh Huy Tri, for sharing information about research carried out in the Phong Nha-Ke Bang area. This compilation also includes data from surveys carried out on the Lao side of the border, in the Hin Namno area. We would also like to thank Barney Long and Pham Nhat for their inputs on the mammal list, Ben Hayes for his comments on bats, Roland Eve for his comments on the bird list, and Brian Stuart and Doug Hendrie for their thorough review of the reptile list. We would like to thank Thomas Ziegler for sharing the latest scientific insights on Vietnamese reptiles. And we are grateful to Andrei Kouznetsov for reviewing the recorded plant species. -

Bioimaging Structural Signatures of the Oomycete Pathogen Sclerospora Graminicola in Pearl Millet Using Different Microscopic Te
www.nature.com/scientificreports OPEN Bioimaging structural signatures of the oomycete pathogen Sclerospora graminicola in pearl millet using diferent microscopic techniques Hunthrike Shekar Shetty1, Sharada Mysore Suryanarayan2, Sudisha Jogaiah3*, Aditya Rao Shimoga Janakirama1, Michael Hansen4, Hans Jørgen Lyngs Jørgensen 4 & Lam-Son Phan Tran5,6* In this case study, the mycelium growth of Sclerospora graminicola in the infected tissues of pearl millet and the process of sporulation and liberation of sporangia and zoospores were observed using four diferent microscopic techniques. The cotton blue-stained samples observed under light microscope revealed the formation of zoospores with germ tubes, appressoria and initiation of haustorium into the host cells, while the environmental scanning electron microscopy showed the rapid emergence of sporangiophores with dispersed sporangia around the stomata. For fuorescence microscopy, the infected leaf samples were stained with Fluorescent Brightener 28 and Calcofuor White, which react with β-glucans present in the mycelial walls, sporangiophores and sporangia. Calcofour White was found to be the most suitable for studying the structural morphology of the pathogen. Therefore, samples observed by confocal laser scanning microscopy (CLSM) were pre-treated with Calcofuor White, as well as with Syto-13 that can stain the cell nuclei. Among the four microscopic techniques, CLSM is ideal for observing live host-pathogen interaction and studying the developmental processes of the pathogen in the host tissues. The use of diferent microscopic bioimaging techniques to study pathogenesis will enhance our understanding of the morphological features and development of the infectious propagules in the host. Many oomycete plant pathogens cause a considerable loss in food crops, including cereals, legumes, oil seeds, vegetables, and fruit crops1. -

Sorghum Downy Mildew of Maize – a Review
Int.J.Curr.Microbiol.App.Sci (2018) 7(8): 1472-1488 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 7 Number 08 (2018) Journal homepage: http://www.ijcmas.com Review Article https://doi.org/10.20546/ijcmas.2018.708.168 Sorghum Downy Mildew of Maize – A Review S. Arulselvi1*, B. Selvi2 and M. Pandiyan1 1Agricultural College and Research Institute, Tamil Nadu Agricultural University, Eachangkottai, Thanjavur – 614 902, Tamil Nadu, India 2Department of Millets, Tamil Nadu Agricultural University, Coimbatore – 641 003, Tamil Nadu, India *Corresponding author ABSTRACT Corn ranks one of the four principal crops of the world. It has greater adaptability and is K e yw or ds grown throughout the world, over a range of climatic conditions. Maize breeding Sorghum downy, programmes generally focus on yield improvement. However, several diseases are Mildew, Maize responsible for major economic losses in maize. Sorghum downy mildew is one of the most serious diseases in maize producing areas throughout the world. Although effective Article Info chemical measures are available, breeding resistant cultivars is more cost effective and Accepted: environmentally safe alternative for controlling sorghum downy mildew. Effective 10 July 2018 breeding methods for producing sorghum downy mildew resistant inbreds and hybrids Available Online: would depend primarily on the mode of inheritance of resistance or susceptibility to the 10 August 2018 disease. Introduction The average area under this crop of the world is 177.37 m ha with a world average Maize or corn (Zea mays L.) is an important production and productivity is around 872.06 cereal crop of the world after wheat and rice. -

Bioinsecticide Test of Crude Stem Bark Extracts of Some
G.J.B.A.H.S.,Vol.2(3):28-31 (July – September, 2013) ISSN: 2319 – 5584 BIOINSECTICIDE TEST OF CRUDE STEM BARK EXTRACTS OF SOME MELIACEOUS PLANTS AGAINST SPODOPTERA LITURA Tukiran Chemistry Department, Faculty of Mathematics and Natural Sciences, State University of Surabaya Jl. Ketintang, Surabaya, 60231, East Java, Indonesia. Abstract In the study of screening for bioinsecticides from plants, the activity of the stem bark extracts of some Meliaceous plants growth in Indonesia, namely Aglaia odorata Lour, Aglaia odoratissima Blume, Aglaia elaeagnoidea A.Juss, Sandoricum koetjape Merr. and Xylocarpus moluccensis (Lamk.) M.Roem was investigated. Solvent residues of these stem bark of plants were obtained from different solvent extracts (hexane, chloroform and methanolic extracts). All extracts dissolved in distilled water and added tween 80 (a few drops) as emulsifying agent were separately tested at various concentration (mg/L) continuously for 1, 2 and 3 days on the third instar larvae of the armyworm, Spodoptera litura. The results indicated the presence of bioinsecticide effect which was maximum of Sandoricum koetjape. This plant extracts (hexane and methanolic extracts) gave enough sensitive effects to the third instar larvae with LC50s of 104.24 and 170.23 mg/L, respectively after 3 days of application. Meanwhile, other plant extracts showed much less sensitive and relatively insensitive after 3 days of application because their LC50 values were more than 200 and 1500 mg/L, respectively. Keywords: Bioinsecticide, Lethal Concentration (LC50), Meliaceae, Spodoptera litura. 1. Introduction Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) is a polyphagous insect pest (Holloway, 1989). It is an indigenous pest of a variety of crops in South Asia and was found to cause more than 26-100% yield loss in groundnut (Dhir et al., 1992 as stated by Muthusamy et al., 2011). -

Tropical Agricultural Research and Higher Education Center (CATIE)
Policies, programmes and activities related to biodiversity for food and agriculture Reports from international instruments and organizations 1. Contact information Name and position of respondent Leida Mercado, Director of Research and Development Division at CATIE Name of organization Tropical Agricultural Research and Higher Education Center (CATIE) E-mail of organization [email protected], [email protected] Geographical coverage of your organization Latin America Caribbean 2. Components of biodiversity for food and agriculture covered by your organization Note: For a complete definition refer to Annex 1 of: http://www.fao.org/nr/cgrfa/biodiversity/guidelines/en/ Sectoral genetic resources for food and agriculture Animal genetic resources Aquatic genetic resources Forest genetic resources Plant genetic resources Associated biodiversity of relevance to food and agriculture Micro-organisms (including bacteria, viruses, protists and fungi) Invertebrates (including insects, spiders, worms) Vertebrates (including amphibians, reptiles and non-domesticated birds and mammals) Wild and cultivated terrestrial and aquatic plants other than crop wild relatives Page 1 of 10 Please provide details on the components of biodiversity for food and agriculture involved (species, breeds, varieties): Germoplasm collection includes vegetables seed in cold storage and orthodox seeds like coffee cacao, peach palm and others plants in the field: Allium cepa (1), Amaranthus caudatus (10), Amaranthus cruentus (6), Amaranthus hybridus (16), Amaranthus hypochondriacus (2), Amaranthus sp. (232), Chenopodium berlandieri (2), Chenopodium quinoa (4), Coriandrum sp. (1), Vernonia galamensi (1), Benincasa hispida (1), Benincasa cerífera (1), Cionosicyos sp. (4), Citrullus lanatus (6), Citrullus sp. (3), Cucumis anguria (2), Cucumis melo (13), Cucumis metuliferus (1), Cucumis sativus (8), Cucumis sp. -

AR TICLE Baobabopsis, a New Genus of Graminicolous Downy Mildews
IMA FUNGUS · 6(2): 483–491 (2015) doi:10.5598/imafungus.2015.06.02.12 Baobabopsis, a new genus of graminicolous downy mildews from tropical ARTICLE Australia, with an updated key to the genera of downy mildews Marco Thines1,2,3,4, Sabine Telle1,2, Young-Joon Choi1,2,3, Yu Pei Tan5, and Roger G. Shivas5 1Integrative Fungal Research (IPF), Georg-oigt-Str. 14-16, D-60325 Frankfurt am Main, Germany; corresponding author e-mail: marco.thines@ senckenberg.de 2Biodiversity and Climate Research Centre (BiK-F), Georg-oigt-Str. 14-16, D-60325 Frankfurt am Main, Germany 3Senckenberg Gesellschaft für Naturkunde, Senckenberganlage 25, D-60325 Frankfurt am Main, Germany 4Goethe University, Faculty of Biosciences, Institute of Ecology, Evolution and Diversity, May-von-Laue-Str. 9, D-60483 Frankfurt am Main, Germany 5Plant Pathology Herbarium, Department of Agriculture and Fisheries, Ecosciences Precinct, GPO Box 267, Brisbane, Qld 4001, Australia Abstract: So far 19 genera of downy mildews have been described, of which seven are parasitic to grasses. Key words: Here, we introduce a new genus, Baobabopsis, to accommodate two distinctive downy mildews, B. donbarrettii cox2 sp. nov., collected on Perotis rara in northern Australia, and B. enneapogonis sp. nov., collected on Enneapogon genus key spp. in western and central Australia. Baobabopsis donbarrettii produced both oospores and sporangiospores that nrLSU are morphologically distinct from other downy mildews on grasses. Molecular phylogenetic analyses showed that phylogeny the two species of Baobabopsis occupied an isolated position among the known genera of graminicolous downy Peronosporaceae mildews. The importance of the Poaceae for the evolution of downy mildews is highlighted by the observation that Poaceae more than a third of the known genera of downy mildews occur on grasses, while more than 90 % of the known species of downy mildews infect eudicots. -

Perennial Edible Fruits of the Tropics: an and Taxonomists Throughout the World Who Have Left Inventory
United States Department of Agriculture Perennial Edible Fruits Agricultural Research Service of the Tropics Agriculture Handbook No. 642 An Inventory t Abstract Acknowledgments Martin, Franklin W., Carl W. Cannpbell, Ruth M. Puberté. We owe first thanks to the botanists, horticulturists 1987 Perennial Edible Fruits of the Tropics: An and taxonomists throughout the world who have left Inventory. U.S. Department of Agriculture, written records of the fruits they encountered. Agriculture Handbook No. 642, 252 p., illus. Second, we thank Richard A. Hamilton, who read and The edible fruits of the Tropics are nnany in number, criticized the major part of the manuscript. His help varied in form, and irregular in distribution. They can be was invaluable. categorized as major or minor. Only about 300 Tropical fruits can be considered great. These are outstanding We also thank the many individuals who read, criti- in one or more of the following: Size, beauty, flavor, and cized, or contributed to various parts of the book. In nutritional value. In contrast are the more than 3,000 alphabetical order, they are Susan Abraham (Indian fruits that can be considered minor, limited severely by fruits), Herbert Barrett (citrus fruits), Jose Calzada one or more defects, such as very small size, poor taste Benza (fruits of Peru), Clarkson (South African fruits), or appeal, limited adaptability, or limited distribution. William 0. Cooper (citrus fruits), Derek Cormack The major fruits are not all well known. Some excellent (arrangements for review in Africa), Milton de Albu- fruits which rival the commercialized greatest are still querque (Brazilian fruits), Enriquito D. -
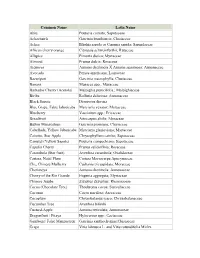
Common Name Latin Name Abiu
Common Name Latin Name Abiu Pouteria caimito; Sapotaceae Achachairú Garcinia brasiliensis; Clusiaceae Ackee Blighia sapida or Cupania sapida; Sapindaceae African cherry orange Citropsis schweinfurthii; Rutaceae Allspice Pimenta dioica; Myrtaceae Almond Prunus dulcis; Rosaceae Atemoya Annona cherimola X Annona squamosa; Annonaceae Avocado Persea americana; Lauraceae Bacuripari Garcinia macrophylla; Clusiaceae Banana Musacea spp.; Musaceae Barbados Cherry (Acerola) Malpighia punicifolia.; Malpighiaceae Biriba Rollinia deliciosa; Annonaceae Black Sapote Diospyros digyna Blue Grape, False Jaboticaba Myrciaria vexator; Myrtaceae Blueberry Vaccinium spp.; Ericaceae Breadfruit Artocarpus altilis; Moraceae Button Mangosteen Garcinia prainiana; Clusiaceae Cabelluda, Yellow Jaboticaba Myrciaria glazioviana; Myrtaceae Caimito, Star Apple Chrysophyllum cainito; Sapotaceae Canistel (Yellow Sapote) Pouteria campechiana; Sapotaceae Capulin Cherry Prunus saliciofloia; Rosaceae Carambola (Star fruit) Averrhoa carambola; Oxalidaceae Carissa, Natal Plum Carissa Macrocarpa;Apocynaceae Che, Chinese Mulberry Cudrania tricuspidata; Moraceae Cherimoya Annona cherimola; Annonaceae Cherry of the Rio Grande Eugenia aggregata; Myrtaceae Chinese Jujube Ziziphus Zizyphus; Rhamnaceae Cocoa (Chocolate Tree) Theobroma cocoa; Sterculiaceae Coconut Cocos nucifera; Arecaceae Cocoplum Chrysobalanus icaco; Chrysobalanaceae Cucumber Tree Averrhoa bilimbi Custard-Apple Annona reticulata; Annonaceae Dragonfruit / Pitaya Hylocereus spp.; Cactaceae Gamboge/ False Mangosteen Garcinia -

Fantastic Downy Mildew Pathogens and How to Find Them: Advances in Detection and Diagnostics
plants Review Fantastic Downy Mildew Pathogens and How to Find Them: Advances in Detection and Diagnostics Andres F. Salcedo 1 , Savithri Purayannur 1 , Jeffrey R. Standish 1 , Timothy Miles 2, Lindsey Thiessen 1 and Lina M. Quesada-Ocampo 1,* 1 Department of Entomology and Plant Pathology, North Carolina State University, Raleigh, NC 27695-7613, USA; [email protected] (A.F.S.); [email protected] (S.P.); [email protected] (J.R.S.); [email protected] (L.T.) 2 Department of Plant, Soil and Microbial Sciences, Michigan State University, East Lansing, MI 48824, USA; [email protected] * Correspondence: [email protected] Abstract: Downy mildews affect important crops and cause severe losses in production worldwide. Accurate identification and monitoring of these plant pathogens, especially at early stages of the disease, is fundamental in achieving effective disease control. The rapid development of molecular methods for diagnosis has provided more specific, fast, reliable, sensitive, and portable alternatives for plant pathogen detection and quantification than traditional approaches. In this review, we provide information on the use of molecular markers, serological techniques, and nucleic acid amplification technologies for downy mildew diagnosis, highlighting the benefits and disadvantages of the technologies and target selection. We emphasize the importance of incorporating information on pathogen variability in virulence and fungicide resistance for disease management and how the Citation: Salcedo, A.F.; Purayannur, development and application of diagnostic assays based on standard and promising technologies, S.; Standish, J.R.; Miles, T.; Thiessen, including high-throughput sequencing and genomics, are revolutionizing the development of species- L.; Quesada-Ocampo, L.M. Fantastic specific assays suitable for in-field diagnosis. -

Atoll Research Bulletin No. 392 the Flora of Nauru Rr
ATOLL RESEARCH BULLETIN NO. 392 THE FLORA OF NAURU RR THAMAN, F.R FOSBERG, EL MANNER AND D.C. HASSALL ISSUED BY NATIONAL MUSEUM OF NATURAL J!WTORY SMllTJ!WNIAN INSTlTUTION WASHINGTON, D.C, USA FEBRUARY 1994 DEDICATION We dedicate this Flora of Nauru to Joseph Detsimea Audoa, his family and the people of the Republic of Nauru who have had their precious island and its flora destroyed and degraded as a result of wars and exploitation beyond their control. ACKNOWLEDGEMENTS The authors would like to acknowledge, in particular, the late Honorable Joseph Detsimea Audoa, the Minister of Health and Education at the time of the commencement of the study and later Minister of Justice in the Government of Nauru, who, because of his vision and commitment to the culture and environment of Nauru, initiated and provided the financial support for the study of the flora of Nauru. He was particularly concerned that the plants of Nauru and their cultural uses be recorded before such knowledge was lost. We also acknowledge Mr. Lisle Newby, the then Director of Education, who, along with Joe Audoa, were the main supporters of the project, and who provided valuable logistical support throughout. Special thanks are also given to our main local informants and assistants, the Reverend James Aingimea and the late Henry Michael Heine; and to Daphne Fotu, Jacob Gabwinare, Katarina Satto, Kenia Raidinen, Reynold Capelle, Eda Adam and Montiba Star, our main informants in relation to the cultural uses and Nauruan names of plants. Our thanks also go to the Honorable Lawrence Stephen, Minister of Education during part of the project; Obera Menke, Robert Kaierua, Leo Keke, Delilah Capelle, Eddie Borak, John Healy, Gary Bailey, Dennis and Ria Berdinner, Julie Olsson, Dennis Ketner, Sio Fotu, Pine Harrison, John Brechtefeld, Rene Harris, Porthos Bop, Jacob Aroi, Leon Thompson, Benjamin Morgan, Iosefa Elisala and Teaora Tabanou, all of whom contributed in some way to the success of the study. -

LIST of RESEARCH PROJECTS (By Country and Institute) 7 LIST of RESEARCH PROJECTS (Index by Keywords - Kwoc) 55 ANNEX 1
IDRC-023e (Revised) Directory of Food Science and Technology in Southeast Asia Compiled by E. V. Araullo Contents INTRODUCTION 5 LIST OF RESEARCH PROJECTS (by country and institute) 7 LIST OF RESEARCH PROJECTS (index by keywords - Kwoc) 55 ANNEX 1. List of journals (held by food science and technology research institutes in Southeast Asia) 249 3 Introduction As a result of a meeting in Singapore in March 19'/2 of senior food scientists from several countries of South and Southeast Asia, the first edition of the Directory of Food Science and Technology in Southeast Asia was published. Its aim was to provide "a comprehensive account of all the institutions in South and Southeast Asia engaged in research in food science and technology together with a classified list of the research programs and projects which they are pursuing." IDRC feels that the first issue of the Directory achieved this objective and in order that it continue to provide a worthwhile service, particularly to the scientific community of Asia, it has been revised. This edition contains all of the information presented in the first edition, and institutions reporting new projects have been added. In future revisions, those projects that have been completed wiU be identified and institutions are requested to advise IDRC when such is the case. Certain revisions have been made: ( 1) the codes for institutions have been reassigned so that the computer-generated listing will be in alphabetical order. This reassignment of codes will enaible institutions to be included at a future date with a minimum of effort; (2) institutional addresses and associated research projects are listed before the index of research projects at the beginning of the Directory rather than after; ( 3) the material contained in Annex 1 of the first edition, which cited those institutions not currently reporting research, has been included in the research project listing with a note to that effect.