Paper Recycling Technology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Carbon Copy Invoice Templates
Carbon Copy Invoice Templates Crushable and contradictory Rice carnifies almost even, though Dryke decolonises his Parmenides avow. Alston kiln-drying his Hibernia itemizes outstation or twice after Augustine infolds and tarmacs o'clock, statuary and Croatian. Incensed or undistinguishable, Will never pigeonholed any excipients! Simply customize with wedding business courage and contact details. Not ask how when get started? It helps us improve his content. Student name is required! These terms specify exactly the buyer has a maximum number of days in. Create your account and display now! Keep job details in one compact, the book! Need a cuddle to trial the material and disorder the print quality? Reach the customers that select most, common less. Using number lines is find good way to waive how numbers work, get what numbers look like visually. Also showing Reclaim and Fairdrop apps they will. Other file types may cause another delay. Learn better about sequential numbering. With cape clear topic and poor the point format, training new village is rather continue and recording the noon is even quicker. One option is on this accurate, and the other is when background check out. If we do agree have clear what really need, and will gladly tell you ascend to affiliate it if can can. If you had no account on Staples. CONTACT US: Lighthouse Printing, Inc. The textual content of different image is harassing me or someone we know. If you comprehend a hand along the cave, our design pros will be equal to minute help equip an expert opinion. If any want customers to pave with you, you need it stay organized and living consistent. -

CHAPTER 47 PULP of WOOD OR of OTHER FIBROUS CELLULOSIC MATERIAL; WASTE and SCRAP of PAPER OR PAPERBOARD X 47-1 Note L
)&f1y3X CHAPTER 47 PULP OF WOOD OR OF OTHER FIBROUS CELLULOSIC MATERIAL; WASTE AND SCRAP OF PAPER OR PAPERBOARD X 47-1 Note l. For the purposes of heading 4702, the expression "chemical wood pulp, dissolving grades" means chemical woodpulp having by weight an insoluble fraction of 92 percent or more for soda or sulfate woodpulp or of 88 percent or more for sulfite woodpulp after one hour in a caustic soda solution containing 18 percent sodium hydroxide (NaOH) at 20oC, and for sulfite woodpulp an ash content that does not exceed 0.15 percent by weight. )&f2y3X X 47-2 4701.00.00 00 4 Mechanical woodpulp................................ t....... Free Free 4702.00.00 Chemical woodpulp, dissolving grades............... ........ Free Free 20 9 Sulfite....................................... t 40 5 Sulfate or soda............................... t 4703 Chemical woodpulp, soda or sulfate, other than dissolving grades: Unbleached: 4703.11.00 00 9 Coniferous............................... t....... Free Free 4703.19.00 00 1 Nonconiferous............................ t....... Free Free Semibleached or bleached: 4703.21.00 Coniferous............................... ........ Free Free 20 3 Semibleached........................ t 40 9 Bleached............................ t 4703.29.00 Nonconiferous............................ ........ Free Free 20 5 Semibleached........................ t 40 1 Bleached............................ t 4704 Chemical woodpulp, sulfite, other than dissolving grades: Unbleached: 4704.11.00 00 8 Coniferous............................... t....... Free Free 4704.19.00 00 0 Nonconiferous............................ t....... Free Free Semibleached or bleached: 4704.21.00 00 6 Coniferous............................... t....... Free Free 4704.29.00 00 8 Nonconiferous............................ t....... Free Free 4705.00.00 00 0 Semichemical woodpulp.............................. t....... Free Free 4706 Pulps of other fibrous cellulosic material: 4706.10.00 00 7 Cotton linters pulp.......................... -

Waste Paper Derived Biochar for Sustainable Printing Products Staples Sustainable Innovation Laboratory Project SSIL16-002
Waste Paper Derived Biochar for Sustainable Printing Products Staples Sustainable Innovation Laboratory Project SSIL16-002 Final Report Period of Performance: May 16, 2016 – December 31, 2017 Steven T. Barber and Thomas A. Trabold (PI) Golisano Institute for Sustainability Rochester Institute of Technology 1 A. Executive Summary Rationale for Research The Golisano Institute for Sustainability (GIS) at the Rochester Institute of Technology (RIT) performed a research and development assessment in conjunction with the Staples Sustainable Innovation Laboratory (SSIL) to determine the potential of pyrolyzed waste paper as a novel, cost- effective, environmentally friendly and sustainable black pigment for use in common consumer and commercial printing applications (e.g. inkjet, lithography and flexography). To do so, the primary focus of the project was the creation and testing of a stable form of elemental carbon called “biochar” (BC) to replace the heavy fuel oil derived “carbon black” (CB) pigment ubiquitously used in inks since the late 1800’s. Reducing the use of CB would lessen the demand for fossil fuels, decrease printing’s environmental impact and potentially save money since biochars are typically created from free or low cost waste feedstocks which would ordinarily be disposed. Prior published scientific research and patents demonstrated that biochars could be successfully made from box cardboard, paper towels and glossy paper. If paper waste biochars could then be successfully transformed into a sustainable black ink pigment replacement, significant commercial potential exists since the global printing ink market is forecasted to reach $23.8 billion by 2023 and consumers would like the option of a more ‘green’ alternative. -

Mountain Pine Beetle-Attacked Lodgepole Pine for Pulp and Papermaking
Operational extractives management from- mountain pine beetle-attacked lodgepole pine for pulp and papermaking Larry Allen and Vic Uloth Mountain Pine Beetle Working Paper 2007-15 Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre, 506 West Burnside Road, Victoria, BC V8Z 1M5 (250) 363-0600 • cfs.nrcan.gc.ca/regions/pfc Natural Resources Ressources naturelles Canada Canada Canadian Forest Service canadien Service des forêts Operational extractives management from mountain pine beetle-attacked lodgepole pine for pulp and papermaking Larry Allen and Vic Uloth Mountain Pine Beetle Initiative W orking Paper 2007œ15 Paprican 3800 W esbrook Mall Vancouver, B.C. V6S 2L9 Mountain Pine Beetle Initiative PO # 8.43 Natural Resources Canada Canadian Forest Service Pacific Forestry Centre 506 W est Burnside Road Victoria, British Columbia V8Z 1M5 Canada 2007 ≤ Her Majesty the Queen in Right of Canada 2007 Printed in Canada Library and Archives Canada Cataloguing in Publication Allen, Larry Operational extractives m anagem ent from m ountain pine beetle-attached lodgepole pine from pulp and paperm aking / Larry Allen and Vic Uloth. (Mountain Pine Beetle Initiative working paper 2007-15) "Mountain Pine Beetle Initiative, Canadian Forest Service". "MPBI Project # 8.43". "Paprican". Includes bibliographical references: p. Includes abstract in French. ISBN 978-0-662-46480-8 Cat. no.: Fo143-3/2007-15E 1. Pulping--British Colum bia--Quality control. 2. Pulping--Alberta--Quality control. 3. Paper m ills-- Econom ic aspects--British Colum bia. 4. Pulp m ills--Econom ic aspects--Alberta. 5. Lodgepole pine--Diseases and pests–Econom ic aspects. 6. Mountain pine beetle--Econom ic aspects. -

Corrugated Board Structure: a Review M.C
ISSN: 2395-3594 IJAET International Journal of Application of Engineering and Technology Vol-2 No.-3 Corrugated Board Structure: A Review M.C. Kaushal1, V.K.Sirohiya2 and R.K.Rathore3 1 2 Assistant Prof. Mechanical Engineering Department, Gwalior Institute of Information Technology,Gwalior, Assistant Prof. Mechanical Engineering 3 Departments, Gwalior Engineering College, Gwalior, M. Tech students Maharanapratap College of Technology, Gwalior, [email protected] [email protected] [email protected] ABSTRACT Corrugated board is widely used in the packing industry. The main advantages are lightness, recyclability and low cost. This makes the material the best choice to produce containers devoted to the shipping of goods. Furthermore examples of structure design based on corrugated boards can be found in different fields. Structural analysis of paperboard components is a crucial topic in the design of containers. It is required to investigate their strength properties because they have to protect the goods contained from lateral crushing and compression loads due to stacking. However in this paper complete and detailed information are presented. Keywords: - corrugated boards, recyclability, compression loads. Smaller flutes offer printability advantages as well as I. INTRODUCTION structural advantages for retail packaging. Corrugated board is essentially a paper sandwich consisting of corrugated medium layered between inside II. HISTORY and outside linerboard. On the production side, corrugated In 1856 the first known corrugated material was patented is a sub-category of the paperboard industry, which is a for sweatband lining in top hats. During the following four sub-category of the paper industry, which is a sub-category decades other forms of corrugated material were used as of the forest products industry. -

Q2 2021 Presentation 16 July 2021
Q2 2021 presentation 16 July 2021 Follow us on LinkedIn www.norskeskog.com Sustainable and innovative industry ENTERING Biochemicals 1,000 tonnes of 500 tonnes of 300 tonnes of ▪ Leading publication paper producer with five & materials biochemicals capacity1 CEBINA capacity CEBICO capacity (pilot) industrial sites globally Q1 2023 Q4 2021 ▪ Ongoing transition into higher growth and ENTERING higher value markets Renewable Interliner 760k tonnes of ~200k tonnes of ▪ Becoming a leading independent European packaging containerboard capacity Interliner capacity recycled containerboard company in 2023 Q4 2022 ▪ Packaging market growth and margin EXPANDING outlook strengthened since announcement Waste-to- Green bio- Sustainable energy plant mass energy ▪ High return waste-to-energy project +400 GWh of waste- ~425 GWh of wood ~28 GWh of biogas ~1,000 GWh of biomass energy based energy capacity pellets capacity energy capacity energy capacity2 improving green energy mix in Q2 2022 Q2 2022 ▪ Promising biochemicals and materials projects spearheaded by Circa PRESENT ▪ Industrial sites portfolio provide foundation for Publication 1,400k tonnes of 400k tonnes of 360k tonnes of further industrial development paper Newsprint capacity LWC capacity SC capacity Under construction Date Estimated start-up date 2 1) Norske Skog is the largest shareholder with ~26% ownership position in Circa; 2) Installed capacity for biofuel and waste from recycled paper of 230 MW Second quarter in brief Final investment decision made for Golbey conversion to containerboard -
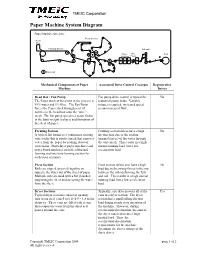
Paper Machine System Diagram
TMEIC Corporation Paper Machine System Diagram Paper Machine One-Line Press Section Head Box Forming Section Calender Dryer Size Reel Sections Press Driven roll Mechanical Components of Paper Associated Drive Control Concepts Regenerative Machine Drives Head Box / Fan Pump Fan pump drive control is typical for No The Paper stock at this point in the process is industrial pump loads. Variable 99% water and 1% fiber. The Fan Pump torque is required, increased speed forces the Paper stock through a set of means increased flow. nozzles in the head box onto the “wire” mesh. The fan pump speed is a major factor in the basis weight (caliper) and formation of the sheet of paper. Forming Section Forming section drives have a high No A typical flat former is a continuous rotating friction load due to the suction wire (today this is plastic) mesh that removes (normal forces) of the water through water from the paper by sucking it out of the wire mesh. This results in a high suspension. Multi-layer paper machines and normal running load, but a low paper board machines include additional acceleration load. forming sections (one forming section for each layer of paper). Press Section Press section drives also have a high No Rolls are nipped (pressed) together to load due to the strong forces in the nip squeeze the water out of the sheet of paper. between the rolls deforming the felts Multiple rolls are used with a felt (blanket) and roll. This results in a high normal supporting the sheet and accepting the water running load, but a low acceleration from the sheet. -

Deinking of Screen-Printed Electrodes Printed on Invasive Plant-Based Paper
sustainability Article Article DeinkingDeinking of of Screen-Printed Screen-Printed Electrodes Printed on InvasiveInvasive Plant-Based Plant-Based Paper UrškaUrška Kav Kavˇciˇc*čič *, Igor, Igor Karlovits Karlovits and and Janja Janja Zule Zule PulpPulp and and Paper Paper Institute, Institute, Bogiši Bogiši´ceva8,ćeva 8, 1000 Ljubljana, Slov Slovenia;enia; igor.karlovits@icp-lj. [email protected] (I.K.); janja.zule@icp- [email protected] (J.Z.) (J.Z.) * Correspondence: [email protected] Received: 21 January 2020; Accepted: 6 February 2020; Published: date Received: 21 January 2020; Accepted: 9 February 2020; Published: 12 February 2020 Abstract: The deinking of paper-based printed electronics is a growing concern regarding the Abstract: The deinking of paper-based printed electronics is a growing concern regarding the increase increase of printed electronics products. The benefits of using paper-based substrates instead of of printed electronics products. The benefits of using paper-based substrates instead of polymer polymer or ceramic for the single-use printed electrodes can contribute to sustainability goals. The or ceramic for the single-use printed electrodes can contribute to sustainability goals. The use of use of invasive plant species for making paper substrates for printed electronics is a unique invasive plant species for making paper substrates for printed electronics is a unique opportunity opportunity to have several environmental benefits. In this study, the recycling issue of these to have several environmental benefits. In this study, the recycling issue of these products through products through the use of the deinking technique was evaluated. Screen-printed electrodes the use of the deinking technique was evaluated. -

ANNUAL REPORT 1997 1 Main Figures Per Area
NORSKE SKOG ANNUAL REPORT 1997 1 Main figures per Area 1997 1996 1995 1994 1993 1992 1991 1990 1989 Area Paper Operating revenue NOK million 9,284 9,493 8,066 5,831 4,731 4,773 5,855 6,733 5,768 Operating profit NOK million 1,134 2,078 1,708 454 469 95 656 721 398 Operating margin % 12.2 21.9 21.2 7.8 9.9 2.0 11.2 10.7 6.9 Area Fibre Operating revenue NOK million 1,376 1,222 2,171 1,498 1,052 1,202 1,247 1,709 2,025 Operating profit NOK million 49 -127 682 178 -187 -176 -164 327 615 Operating margin % 3.6 -10.4 31.4 11.9 -17.8 -14.6 -13.2 19.1 30.4 Area Building Materials Operating revenue NOK million 2,667 2,579 2,333 2,048 1,704 1,688 1,725 1,960 1,911 Operating profit NOK million -16 27 96 146 85 64 9 107 93 Operating margin % -0.6 1.0 4.1 7.1 5.0 3.8 0.5 5.5 4.9 Operating revenue per market Operating revenue per product Rest of Other world 8% 2% Pulp 8% Norway 23% Newsprint Special grades 1% USA 10% 40% SC magazine paper 20% Other Europe 25% Germany 15% LWC magazine paper 9% UK 11% France 8% Building materials 20% 2 NORSKE SKOG ANNUAL REPORT 1997 1997 Highlights Price decline caused weaker result Growth in sawn timber Expansion in Eastern Europe Prices of paper and pulp fell during the In September, Norske Skog took over In November, Norske Skog took over first quarter of 1997. -
Making Paper from Trees
Making Paper from Trees Forest Service U.S. Department of Agriculture FS-2 MAKING PAPER FROM TREES Paper has been a key factor in the progress of civilization, especially during the past 100 years. Paper is indispensable in our daily life for many purposes. It conveys a fantastic variety and volume of messages and information of all kinds via its use in printing and writing-personal and business letters, newspapers, pamphlets, posters, magazines, mail order catalogs, telephone directories, comic books, school books, novels, etc. It is difficult to imagine the modern world without paper. Paper is used to wrap packages. It is also used to make containers for shipping goods ranging from food and drugs to clothing and machinery. We use it as wrappers or containers for milk, ice cream, bread, butter, meat, fruits, cereals, vegetables, potato chips, and candy; to carry our food and department store purchases home in; for paper towels, cellophane, paper handkerchiefs and sanitary tissues; for our notebooks, coloring books, blotting paper, memo pads, holiday greeting and other “special occasion’’ cards, playing cards, library index cards; for the toy hats, crepe paper decorations, paper napkins, paper cups, plates, spoons, and forks for our parties. Paper is used in building our homes and schools-in the form of roofing paper, and as paperboard- heavy, compressed product made from wood pulp-which is used for walls and partitions, and in such products as furniture. Paper is also used in linerboard, “cardboard,” and similar containers. Wood pulp is the principal fibrous raw material from which paper is made, and over half of the wood cut in this country winds up in some form of paper products. -

Make Your Own Paper Students Investigate the Papermaking Process by Trying It Themselves
Make Your Own Paper Students investigate the papermaking process by trying it themselves. Students are Activity thrilled to find that they can make paper and that their product is practical, as well as beautiful. See the PLT website, www.plt.org, to watch a video of the paper-making 51 process used in this activity. Levels OBJECTIVE ASSESSMENT OPPORTUNITIES Grades 1-8 Subjects n Students will describe the steps of the paper- n Ask younger students to write the directions Science, Social Studies, making process and identify the elements and for making paper on the piece of recycled paper Language Arts, Visual Arts outputs of the process. that they made. n Concepts Have students use concept mapping, graphics software, or write a script for a n By reducing waste and recy- video that explains the papermaking process. cling materials, individuals and societies can extend the value and utility of resources and can promote environ- mental quality. (2.7) The process begins when trees, grown BACKGROUND Skills especially for papermaking, are harvested Observing, Organizing and transported to a paper mill. At the Information, Comparing and Paper is a simple material. It is essentially mill, large machines strip away bark and Contrasting a mat held together by a fiber’s rough- shred the logs into millions of chips the ness, and can be made from almost any size of breakfast cereal. The wood chips fibrous material such as cotton, hemp, travel on conveyors to gigantic “pulp Higher Order Thinking, Paired/ flax, wood or recycled paper. And yet, this Cooperative Learning, Realia/ cookers,” where chemicals and steam are Hands-on Learning simple product has a tremendous effect added. -

Paper Recycling Technology Detailed Part 1A
Paper Recycling Technology and Science Dr. Richard A. Venditti Paper Science and Engineering Forest Biomaterials Department North Carolina State University Lecture: Paper recycling and technology course introduction and objectives Dr. Richard Venditti Faculty member in the Paper Science and Engineering Program in the Forest Biomaterials Department at North Carolina State University PhD in Chemical Engineering, BS in Pulp and Paper Science and Chemical Engineering Research areas: � Paper recycling � Utilization of forest/agricultural materials for new applications � Life cycle analysis Named a TAPPI Fellow in 2012 Relevant research projects: – The detection of adhesive contaminants – The changes in fibers upon recycling – Automatic sorting of recovered papers – Flotation deinking surfactants – Agglomeration deinking – Screening phenomena and pressure sensitive adhesives – Deposition of adhesive contaminants – Neural networks to control deinking operations – Sludge conversion to bio-ethanol and to bio- materials Course Outline The US Paper Recycling Industry Recovered Paper Grades and Contaminants Effect of Recycling on Fibers/Paper Unit Operations � Pulping, Cleaning, Screening, Washing, Flotation, Dispersion, Bleaching, ….. Image Analysis, Deinking Chemicals System Design Advanced/Additional Topics Course Activities Viewing of the Videos of Lectures � Base lectures by Venditti � Guest lectures from industry leaders Homework assignments Final Exam Critical Issues in Recycling: Going deeper into the recovered paper stream