Humoral Immune Response Evaluation in Horses Vaccinated with Recombinant Clostridium Perfringens Toxoids Alpha and Beta for 12 Months
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
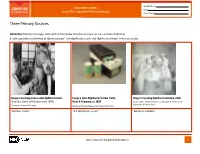
FROM DNA to BEER Date Lesson Plan: Acquired and Passive Immunity Class Period
Student Name FROM DNA TO BEER Date Lesson Plan: Acquired and Passive Immunity Class Period Three Primary Sources Instruction: Examine the images and read their titles below. Based on what you can learn and infer from them: 1. Write your inferred definition of “diphtheria toxin,” “anti-diphtheritic serum,” and “diphtheria antitoxin” in the table below. Image 1. Injecting a horse with diphtheria toxin, Image 2. Anti-Diphtheritic Serum, Parke, Image 3. Injecting diphtheria antitoxin, 1895 New York City Health Department, 1940s Davis & Company, ca. 1898 Courtesy The Historical Medical Library of The College of Physicians of Philadelphia Courtesy Library of Congress Courtesy National Museum of American History “diphtheria toxin”: “anti-diphtheritic serum”: “diphtheria antitoxin”: http://www.nlm.nih.gov/fromdnatobeer 1 Student Name FROM DNA TO BEER Date Lesson Plan: Acquired and Passive Immunity Class Period Three Primary Sources 2. Describe or draw how the three images may be related. http://www.nlm.nih.gov/fromdnatobeer 2 FROM DNA TO BEER Lesson Plan: Acquired and Passive Immunity Teacher’s Three Primary Sources Instruction: Examine the images and read their titles below. Based on what you can learn and infer from them: 1. Write your inferred definition of “diphtheria toxin,” “anti-diphtheritic serum,” and “diphtheria antitoxin” in the table below. Image 1. Injecting a horse with diphtheria toxin, Image 2. Anti-Diphtheritic Serum, Parke, Image 3. Injecting diphtheria antitoxin, 1895 New York City Health Department, 1940s Davis & Company, -

(ACIP) General Best Guidance for Immunization
Appendix 1: Glossary Adverse event. An undesirable medical condition that occurs following vaccination which might be truly caused by a vaccine, or it might be pure coincidence. Adverse reaction. An undesirable medical condition that has been demonstrated to be caused by a vaccine. Evidence for the causal relation is usually obtained through randomized clinical trials, controlled epidemiologic studies, isolation of the vaccine strain from the pathogenic site, or recurrence of the condition with repeated vaccination (i.e., rechallenge); synonyms include side effect and adverse effect. Adjuvant. A vaccine component distinct from the antigen that enhances the immune response to the antigen. Antitoxin. A solution of antibodies against a toxin. Antitoxin can be derived from either human (e.g., tetanus immune globulin) or animal (usually equine) sources (e.g., diphtheria and botulism antitoxin). Antitoxins are used to confer passive immunity and for treatment. Hyperimmune globulin (specific). Special preparations obtained from blood plasma from donor pools preselected for a high antibody content against a specific antigen (e.g., hepatitis B immune globulin, varicella-zoster immune globulin, rabies immune globulin, tetanus immune globulin, vaccinia immune globulin, cytomegalovirus immune globulin, botulism immune globulin). Immune globulin. A sterile solution containing antibodies, which are usually obtained from human blood. It is obtained by cold ethanol fractionation of large pools of blood plasma and contains 15%-18% protein. Intended for intramuscular administration, immune globulin is primarily indicated for routine maintenance of immunity among certain immunodeficient persons and for passive protection against measles and hepatitis A. General Best Practice Guidelines for Immunization: Appendix 1: Glossary 189 Immunobiologic. Antigenic substances (e.g., vaccines and toxoids) or antibody- containing preparations (e.g., globulins and antitoxins) from human or animal donors. -

Diphtheria. In: Epidemiology and Prevention of Vaccine
Diphtheria Anna M. Acosta, MD; Pedro L. Moro, MD, MPH; Susan Hariri, PhD; and Tejpratap S.P. Tiwari, MD Diphtheria is an acute, bacterial disease caused by toxin- producing strains of Corynebacterium diphtheriae. The name Diphtheria of the disease is derived from the Greek diphthera, meaning ● Described by Hippocrates in ‘leather hide.’ The disease was described in the 5th century 5th century BCE BCE by Hippocrates, and epidemics were described in the ● Epidemics described in 6th century AD by Aetius. The bacterium was first observed 6th century in diphtheritic membranes by Edwin Klebs in 1883 and cultivated by Friedrich Löffler in 1884. Beginning in the early ● Bacterium first observed in 1900s, prophylaxis was attempted with combinations of toxin 1883 and cultivated in 1884 and antitoxin. Diphtheria toxoid was developed in the early ● Diphtheria toxoid developed 7 1920s but was not widely used until the early 1930s. It was in 1920s incorporated with tetanus toxoid and pertussis vaccine and became routinely used in the 1940s. Corynebacterium diphtheria Corynebacterium diphtheriae ● Aerobic gram-positive bacillus C. diphtheriae is an aerobic, gram-positive bacillus. ● Toxin production occurs Toxin production (toxigenicity) occurs only when the when bacillus is infected bacillus is itself infected (lysogenized) by specific viruses by corynebacteriophages (corynebacteriophages) carrying the genetic information for carrying tox gene the toxin (tox gene). Diphtheria toxin causes the local and systemic manifestations of diphtheria. ● Four biotypes: gravis, intermedius, mitis, and belfanti C. diphtheriae has four biotypes: gravis, intermedius, mitis, ● All isolates should be tested and belfanti. All biotypes can become toxigenic and cause for toxigenicity severe disease. -

Current Medical Literature American J. Digestive Diseases, Fort Wayne, Ind. Am. J. Roentgenol. & Rad. Therapy, Springfield
American Journal of Public Health, New York Current Medical Literature 33:925-1042 (Aug.) 1943 National Board of Health 1879-1883. W. G. Smillie.—p. 925. Preventive Medicine Program of United States Army. J. S. Simmons. AMERICAN —p. 931. Home Methods and Their Effect on Quality The Association to of Drying Palatability, Cooking library lends periodicals members the Association and Nutritive Value of Foods. Esther L. 941. and to Batchelder.—p. individual subscribers in continental United States and Canada Blood and Malaria Parasite Staining with Eosin Azure Méthylène Blue for a of be borrowed a time. period three days. Three journals may at Methods. R. D. 948. Periodicals are available from 1933 to date. for issues Lillie.—p. Requests of Radio Habits of Attend earlier date cannot be filled. should be Listening Mothers Who Well Baby Clinics. Requests accompanied by L. Murray and C. E. 952. cover if one and 18 if three Margaret Turner.—p. stamps to postage (6 cents cents periodicals Surveys of Nutrition of Populations: 2. Protein Nutrition of Rural are requested). Periodicals the American Medical Asso¬ published by Population in Middle Tennessee. J. B. E. W. ciation are not available for but can be on Youmans, Patton, lending supplied purchase W. R. Ruth Kern and Ruth 955. order. as a rule are the of can be Sutton, Steinkamp.—p. Reprints property authors and Field for Health Education Personnel. Minnie obtained for permanent possession only from them. Experience Krueger Oed. —p. 965. Titles marked with an asterisk (*) are abstracted below. Dehydration Procedures and Their Effect on Vitamin Retention. -

Identification of a Toxin–Antitoxin System That Contributes to Persister Formation by Reducing NAD in Pseudomonas Aeruginosa
microorganisms Article Identification of a Toxin–Antitoxin System That Contributes to Persister Formation by Reducing NAD in Pseudomonas aeruginosa Jingyi Zhou, Shouyi Li, Haozhou Li, Yongxin Jin, Fang Bai , Zhihui Cheng and Weihui Wu * State Key Laboratory of Medicinal Chemical Biology, Key Laboratory of Molecular Microbiology and Technology of the Ministry of Education, Department of Microbiology, College of Life Sciences, Nankai University, Tianjin 300071, China; [email protected] (J.Z.); [email protected] (S.L.); [email protected] (H.L.); [email protected] (Y.J.); [email protected] (F.B.); [email protected] (Z.C.) * Correspondence: [email protected] Abstract: Bacterial persisters are slow-growing or dormant cells that are highly tolerant to bactericidal antibiotics and contribute to recalcitrant and chronic infections. Toxin/antitoxin (TA) systems play important roles in controlling persister formation. Here, we examined the roles of seven predicted type II TA systems in the persister formation of a Pseudomonas aeruginosa wild-type strain PA14. Overexpression of a toxin gene PA14_51010 or deletion of the cognate antitoxin gene PA14_51020 increased the bacterial tolerance to antibiotics. Co-overexpression of PA14_51010 and PA14_51020 or simultaneous deletion of the two genes resulted in a wild-type level survival rate following antibiotic Citation: Zhou, J.; Li, S.; Li, H.; treatment. The two genes were located in the same operon that was repressed by PA14_51020. Jin, Y.; Bai, F.; Cheng, Z.; Wu, W. We further demonstrated the interaction between PA14_51010 and PA14_51020. Sequence analysis Identification of a Toxin–Antitoxin revealed that PA14_51010 contained a conserved RES domain. -

Vaccinating Adults: a Step-By-Step Guide Immunization Action Coalition • • 124124 Appendix B: Glossary
appendix b Glossary Adapted from www.cdc.gov/vaccines/terms/glossary.html Acellular vaccine: A vaccine containing partial cellular Antibody: A protein found in the blood that is produced in material as opposed to complete cells. response to foreign substances (such as bacteria or viruses) invading the body. Antibodies protect the body Acquired Immune Deficiency Syndrome (AIDS): A medical from disease by binding to these organisms and condition where the immune system cannot function destroying them. properly and protect the body from disease. As a result, the body cannot defend itself against infections (like Antigen: A foreign substance (such as bacteria or virus) in pneumonia). AIDS is caused by the Human Immuno - the body that is capable of causing disease. The pres - deficiency Virus (HIV). This virus is spread through direct ence of antigen in the body triggers an immune response, contact with the blood and body fluids of an infected usually the production of antibodies. individual. High risk activities include unprotected sex - Antitoxin: Antibodies capable of destroying toxins gener - ual intercourse and intravenous drug use (sharing ated by microorganisms, including viruses and bacteria. needles). There is no cure for AIDS; however, research efforts are ongoing to develop a vaccine. Antiviral: Literally “against-virus” – any medicine capable of destroying or weakening a virus. Active immunity: The production of antibodies against a specific disease by the immune system. Active immunity Arthralgia: Joint pain. can be acquired in two ways, either by contracting the Arthritis: A medical condition characterized by inflammation disease or through vaccination. Active immunity is usu - of the joints which results in pain and difficulty moving. -

Diphtheria Antitoxin
Diphtheria Antitoxin: Market Update UNICEF Supply Division February 2017 0 Diphtheria Antitoxin Market Update February 2017 1. Summary Following the World Health Organization’s (WHO) Strategic Advisory Group of Experts (SAGE) meeting in October 2016, having expressed concern over the reported lack of diphtheria antitoxin (DAT) serum and recommendations to resolve global shortages,1 UNICEF shares current information on DAT serum supply availability, and its recommendations for future supply to meet emergency requirements. DAT supply availability from manufacturers has been limited for many years. UNICEF does not anticipate any change to this situation, and expects continued shortage through 2017. Countries should continue to monitor closely their DAT stock inventories and take appropriate and early actions to replenish, due to the limited supply availability. UNICEF will work with WHO and the European Centre for Disease Prevention and Control (ECDC), to monitor supply availability and review quality assurance standards, as well as seek additional options for supply. 2. Background Diphtheria is a potentially acute bacterial disease caused by the toxin from Corynebacterium diphtheria. Most cases are asymptomatic or relatively mild. However, the disease can affect the upper respiratory tract and heart, and lead to high case fatality rates.2 Symptoms include weakness, sore throat, fever, swollen neck glands, as well as breathing and swallowing difficulty. Diphtheria spreads through direct contact and droplet transmission from infected -

(DAT) for Suspected Diphtheria Cases
Expanded Access Investigational New Drug (IND) Application Protocol: Use of Diphtheria Antitoxin (DAT) for Suspected Diphtheria Cases BB-IND 11184 Protocol CDC IRB # 4167 Version Number 8.0 March 9, 2020 IND Sponsored by Centers for Disease Control and Prevention (CDC) This expanded access IND protocol for DAT from the CDC contains information intended to guide treating clinicians regarding the use of investigational DAT and should only be used for the stated purpose and scope of the IND protocol in accordance with the applicable IND regulatory requirements. This document, and the contents within the document, should not be altered or reproduced without prior authorization from the CDC. PROGRAM CONTACT INFORMATION Principal Investigator: Anna M. Acosta, MD Meningitis and Vaccine-Preventable Diseases Branch Division of Bacterial Diseases National Center for Immunization and Respiratory Diseases Centers for Disease Control and Prevention 1600 Clifton Road NE, Mailstop H24-6 Atlanta, GA 30329 Office: (404) 639-1951; Fax: (404) 679-5072 E-mail: [email protected] Regulatory Affairs: CAPT Yon Yu, Pharm.D. Regulatory Affairs and Clinical Guidelines Team Lead Division of Preparedness and Emerging Infections National Center for Emerging and Zoonotic Infectious Diseases Centers for Disease Control and Prevention 1600 Clifton Road NE, Mailstop H24-11 Atlanta, GA 30329 Office: (404) 639-3046 E-mail: [email protected] BB-IND 11184 Diphtheria Antitoxin (DAT) Protocol CDC IRB #4167 Version 8.0 Page 1 March 9, 2020 TABLE OF CONTENTS Page 1.0 OBJECTIVE ........................................................................................................................................ -

What Is the Target Group for Antitoxin Treatment to Prevent Clostridioides
What is the target group for antitoxin treatment to prevent Clostridioides difficile infection? Dr Tanya M Monaghan Clinical Associate Professor & Honorary Consultant Gastroenterologist NIHR Nottingham Digestive Diseases Biomedical Research Centre University of Nottingham, UK ESCMID eLibrary © by author Transparency declaration • I am a Consultant advisor to CHAIN Biotechnology • I co-supervise a BBSRC iCASE DTP PhD-funded student with industrial partner CHAIN Biotechnology ESCMID eLibrary © by author Presentation overview • Toxins in disease pathogenesis • Antitoxin strategies • Role of humoral immunity in host defence • Evidence review • Likely place in therapy • Unanswered questions and future considerations ESCMID eLibrary © by author Structure of C. difficile toxins A (TcdA) and B (TcdB) AktoriesESCMIDK et al 2017 Annu. Rev. Microbiol 71; 281-307 eLibrary © by author Toxin-induced CDI pathogenesis ESCMID eLibraryAktories K et al 2017 Annu. Rev. Microbiol 71; 281-307 © by author ChandrasekaranESCMIDR at al 2017 FEMS Microbial Rev; 41: 723 -750eLibrary © by author Antitoxin antibody-mediated therapies for C. difficile infection Passive immunotherapies Active immunotherapies/toxin- • Systemic: based vaccines • Humanised antitoxin mAbs • Systemic: • Polyclonal IVIg • PF-06425090 (Pfizer; phase III) • Genetically and chemically detoxified • Oral TcdA and TcdB • IgAbulin • VLA84 (Valneva; phase II) • Hyperimmune bovine Ig • Recombinant chimeric protein concentrate linking binding domains of TcdA and • Mucomilk (polyclonal-antibody TcdB -

TEE TRAITSFER of ANTIBODY from MCTEER to FOETUS in the Guirea-FIG Thesis Submitted for the Decree of Doctor of Philosophy Of
TEE TRAITSFER OF ANTIBODY FROM MCTEER TO FOETUS IN THE GUIrEA-FIG Thesis submitted for the Decree of Doctor of Philosophy of The University of Leeds by Naha Raouf Al-Y,, a jdi B. Sc. (Baghdad), M. Sc. (Tallahassee, Florida). Department of Bacteriology University of Leeds October 1965 BEST COPY AVAILABLE Variable print quality CONTAINS PULLOUTS CC1' i ETTS Page AC1 'CvLMGMENiS 1 I: TOflUCTIQ:; """"""""""""""".................. Etd 4 LI i EZATLRE RE'JI ............................ Postnatal (colostral) transfer of immunity.. 4 46 Pro- and postnatal transfer of i: ir^unity .... 61 Prenatal transfer of immunity .............. 98 Miscellaneous .............................. '11,TTH0D S 99 E PEitilt'ENTAL ......................... 140 ? ESL'LTS ...................................... DISCL'SSICT: 171 .......:........................... Sumay 193 ...................................... REFERENCES 196 ................................... ACI: r. OWLEDGMENTS I wish to express my thanks and gratitude to Professor C. L. Oakley, F. R. S., for suggesting the problem investigated, and for his interest, generous help and advice throughout the course of this study and the preparation of this manuscript. I would like to thank him for his supply of heterologous antitoxins essential for this study and for his pepsin-di`estion of the guinea-pig tetanus antitoxin. I would like to thank Professor F. W. Rogers Brambell, F. R. S., for allowing me to visit his laboratory and Dr W. A. 'r ernnings for demonstrating the technique of intra-uterine injections and collection of samples in the rabbit. I would like to thank all members of the Department of Bacteriology and especially Mrs Y. Robinson, for their help in many aspects of my work. I wish to thank riss E. M. Reed and her staff at the Leeds Medical School Library for their co-operation and 1 rs M. -
Antitoxin System in the Pvir Plasmid of Campylobacter Jejuni Rocky Damodar Patil Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2011 Identification and characterization of a toxin- antitoxin system in the pVir plasmid of Campylobacter jejuni Rocky Damodar Patil Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Veterinary Preventive Medicine, Epidemiology, and Public Health Commons Recommended Citation Patil, Rocky Damodar, "Identification and characterization of a toxin-antitoxin system in the pVir plasmid of Campylobacter jejuni" (2011). Graduate Theses and Dissertations. 10297. https://lib.dr.iastate.edu/etd/10297 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Identification and characterization of a toxin-antitoxin system in the pVir plasmid of Campylobacter jejuni by Rocky Damodar Patil A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Microbiology Program of Study Committee: Qijing Zhang, Major Professor Bryan Bellaire Aubrey Mendonca Iowa State University Ames, Iowa 2011 Copyright © Rocky Damodar Patil, 2011. All rights reserved. ii TABLE OF CONTENTS ABSTRACT ………………………………………………………………………………... iii CHAPTER 1. GENERAL INTRODUCTION -

Diphtheria: Questions and Answers Q&A Information About the Disease and Vaccines
Diphtheria: Questions and Answers Q&A information about the disease and vaccines What causes diphtheria? How do I know if someone has diphtheria? Diphtheria is caused by a bacterium, Corynebacterium The diagnosis of diphtheria can only be confirmed after diphtheriae. The actual disease is caused when the bacte- a physician takes a small sample of infected material ria release a toxin, or poison, into a person's body. from the patient's throat (or other site) and has the sample tested in a laboratory. But because this disease How does diphtheria spread? progresses quickly, treatment usually should begin Diphtheria bacteria live in the mouth, throat, and nose based on the health professional's assessment of the of an infected person and can be passed to others patient. by coughing or sneezing. Occasionally, transmission occurs from skin sores or through articles soiled with Is there a treatment for diphtheria? oozing from sores of infected people. Diphtheria is treated with both antibiotics and with diphtheria antitoxin. Diphtheria antitoxin is produced How long does it take to show signs of diphtheria in horses and was first used in the United States in after being exposed? 1891. Antitoxin does not get rid of toxin that is already The incubation period is short: 2–5 days, with a range attached to the body's tissues, but will neutralize any of 1–10 days. circulating poison and will prevent the disease from getting worse. The patient should be tested for sensi- What are the symptoms of diphtheria? tivity to this antitoxin before it is given. Early symptoms of diphtheria may mimic a cold with a How common is diphtheria in the United States? sore throat, mild fever, and chills.