Diphtheria Antitoxin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Energy Efficiency And
Progress with The Energy Policy Review: A Perspective OIES Seminars 7 October 2003 John Bower Overview What the White Paper Said Reality Dawns An alternative 20:20 Vision John Bower Progress on UK Energy White Paper 2 What the White Paper Said UKEWP refocused energy policy away from a UK driven liberalisation agenda… GOALS AND POLICIES 1. Reduce CO2 emissions by 60% by 2050 Reduce amount of energy we consume Central to future market and policy will be emissions trading Raise efficiency standards in home appliances and housing Encourage low carbon fuels and renewables through grants and subsidy 2. Maintain reliability of energy supplies Right infrastructure / regulatory systems in UK and liberalisation of Europe Pursue regional stability and economic reform in producing areas Promote understanding of markets and conditions for FDI in producing areas Forward prices will signal the need for investment Improve contingency planning in dealing with major incidents John Bower Progress on UK Energy White Paper 3 What the White Paper Said …. towards an EU driven multifaceted agenda GOALS AND POLICIES 3. Promote competitive markets in UK and beyond Raise rate of sustainable economic growth Support business and competitiveness through reliable / affordable energy Encourage firms to innovate, reduce cost, deliver better goods and services Use market based instruments to deliver policy goals Work with business to prepare them for the low carbon economy of the future 4. Ensure that every home is adequately and affordably heated Reduce poverty by lowering prices and raising social security payments Improve quality of housing stock via insulation and energy efficiency grants John Bower Progress on UK Energy White Paper 4 What the White Paper Said UKEWP relied on carbon trading and uneconomic/unproven technology… ENERGY SYSTEM IN 2020 1. -
FROM DNA to BEER Date Lesson Plan: Acquired and Passive Immunity Class Period
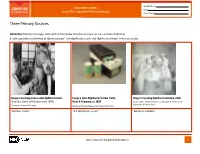
Student Name FROM DNA TO BEER Date Lesson Plan: Acquired and Passive Immunity Class Period Three Primary Sources Instruction: Examine the images and read their titles below. Based on what you can learn and infer from them: 1. Write your inferred definition of “diphtheria toxin,” “anti-diphtheritic serum,” and “diphtheria antitoxin” in the table below. Image 1. Injecting a horse with diphtheria toxin, Image 2. Anti-Diphtheritic Serum, Parke, Image 3. Injecting diphtheria antitoxin, 1895 New York City Health Department, 1940s Davis & Company, ca. 1898 Courtesy The Historical Medical Library of The College of Physicians of Philadelphia Courtesy Library of Congress Courtesy National Museum of American History “diphtheria toxin”: “anti-diphtheritic serum”: “diphtheria antitoxin”: http://www.nlm.nih.gov/fromdnatobeer 1 Student Name FROM DNA TO BEER Date Lesson Plan: Acquired and Passive Immunity Class Period Three Primary Sources 2. Describe or draw how the three images may be related. http://www.nlm.nih.gov/fromdnatobeer 2 FROM DNA TO BEER Lesson Plan: Acquired and Passive Immunity Teacher’s Three Primary Sources Instruction: Examine the images and read their titles below. Based on what you can learn and infer from them: 1. Write your inferred definition of “diphtheria toxin,” “anti-diphtheritic serum,” and “diphtheria antitoxin” in the table below. Image 1. Injecting a horse with diphtheria toxin, Image 2. Anti-Diphtheritic Serum, Parke, Image 3. Injecting diphtheria antitoxin, 1895 New York City Health Department, 1940s Davis & Company, -

MI4A Vaccine Purchase Data for Countries This Note Is Intended to Guide Countries in Their Use of the MI4A Vaccine Purchase Data
MI4A Vaccine Purchase Data for Countries This note is intended to guide countries in their use of the MI4A vaccine purchase data. What data is available? new vaccine introductions, but also for product switches – and to understand the status of product The WHO vaccine market intelligence database registrations. Fifty-six Member States responded compiles vaccine purchase (product, price and to these new questions. procurement) data as reported by countries via the WHO/UNICEF Joint Reporting Form (JRF) over the This information is essential to WHO’s efforts to period of 2013–2018. While country names are not improve global market forecasts and inform displayed, data is publicly available on the MI4A suppliers’ and global policy-makers’ investment website and provides information by country decisions. WHO encourages all countries to characteristics (e.g. region, income group…) by report information on new introduction plans and vaccine and by year of purchase, along with price planned product changes as well as to respond to per dose (USD), procurement mechanism and questions related to registration. annual volumes. 1 The number of countries reporting vaccine How can my country use this data? purchase data through the JRF continues to Countries can use the vaccine purchase data in increase year after year, with 182 Member States several different ways, such as to: (93%) reporting some vaccine procurement information in 2019 – including 158 (81%) fully • Understand how the price of vaccines in their reporting on vaccine purchase data. Price reporting immunization schedule relate to other this year marks a 3% increase from last year and products on the market, or compare the more than triple the reporting from 2016. -

Contribution of Podoviridae and Myoviridae Bacteriophages
www.nature.com/scientificreports OPEN Contribution of Podoviridae and Myoviridae bacteriophages to the efectiveness of anti‑staphylococcal therapeutic cocktails Maria Kornienko1*, Nikita Kuptsov1, Roman Gorodnichev1, Dmitry Bespiatykh1, Andrei Guliaev1, Maria Letarova2, Eugene Kulikov2, Vladimir Veselovsky1, Maya Malakhova1, Andrey Letarov2, Elena Ilina1 & Egor Shitikov1 Bacteriophage therapy is considered one of the most promising therapeutic approaches against multi‑drug resistant bacterial infections. Infections caused by Staphylococcus aureus are very efciently controlled with therapeutic bacteriophage cocktails, containing a number of individual phages infecting a majority of known pathogenic S. aureus strains. We assessed the contribution of individual bacteriophages comprising a therapeutic bacteriophage cocktail against S. aureus in order to optimize its composition. Two lytic bacteriophages vB_SauM‑515A1 (Myoviridae) and vB_SauP‑ 436A (Podoviridae) were isolated from the commercial therapeutic cocktail produced by Microgen (Russia). Host ranges of the phages were established on the panel of 75 S. aureus strains. Phage vB_ SauM‑515A1 lysed 85.3% and vB_SauP‑436A lysed 68.0% of the strains, however, vB_SauP‑436A was active against four strains resistant to vB_SauM‑515A1, as well as to the therapeutic cocktail per se. Suboptimal results of the therapeutic cocktail application were due to extremely low vB_SauP‑436A1 content in this composition. Optimization of the phage titers led to an increase in overall cocktail efciency. Thus, one of the efective ways to optimize the phage cocktails design was demonstrated and realized by using bacteriophages of diferent families and lytic spectra. Te wide spread of multidrug-resistant (MDR) bacterial pathogens is recognized by the World Health Organi- zation (WHO) as a global threat to modern healthcare1. -

List of Registred Drugs in Armenia (01.03.2017-31.03.2017)
LIST OF REGISTRED DRUGS IN ARMENIA (01.03.2017-31.03.2017) International nonproprietary name Dose and Registration Term of Legal Status N Trade name Drug form Manufacturer Country ATC1 code License holder (generic) or active packaging number registration for Supply ingredients name Eli Lilly Regional Lilly France S.A.S., Operations GmbH., solution for 100IU/ml, Zone Industrielle, 2 17.03.2017 1 Abasaglar insulin glargine France A10AE04 16535 PoM2 Koelblgasse 8-10, injection 3ml cartridges (5) rue du Colonel Lilly, 17.03.2022 1030, Vienna, 67640 Fegersheim Austria Olainfarm JSC, 5 300mg, Olainfarm JSC, 5 06.03.2017 Rupnicu Str., 2 Adaptol mebicar capsules hard in blister (20/2x10/, Rupnicu Str., Olaine, Latvia N06BX21 16440 PoM 06.03.2022 Olaine, LV-2114, 30/3x10/, 40/4x10/) LV-2114 Latvia Olainfarm JSC, 5 Olainfarm JSC, 5 500mg, 06.03.2017 Rupnicu Str., 3 Adaptol mebicar tablets Rupnicu Str., Olaine, Latvia N06BX21 16441 PoM in blister (20/2x10/) 06.03.2022 Olaine, LV-2114, LV-2114 Latvia 10mg, Pharmstandard- OTCPharm PJSC, fabomotizole in blisters Leksredstva JSC, 27.03.2017 123317, Moscow, 4 Afobazol (fabomotizole tablets (30/1x30/, 60/3x20/, 305022, Kursk, Russia N05BX04 14729/2 PoM 30.07.2020 Testovskaya str., 10, dihydrochloride) 60/2x30/, 90/3x30/, Agregatnaya 2nd str., Russia 120/4x30/) 1a/18 Gedeon Richter 15mg/g, Gedeon Richter PLC, 17.03.2017 PLC, Gyomroi ut 5 Airtal aceclofenac cream 60g aluminium Gyomroi ut 19-21, Hungary M01AB16 16514 OTC3 17.03.2022 19-21, 1103 tube 1103 Budapest Budapest, Hungary 1 Gedeon Richter PLC, Gyomroi ut 19-21, 1103 Budapest - batch releaser, Industrias Gedeon Richter Farmaceuticas powder for oral 100mg, 17.03.2017 PLC, Gyomroi ut 6 Airtal aceclofenac Almirall S.L., Ctra. -

Annex 2 Composition of Oral and Injectable Estrogen–Progestogen Contraceptives
ANNEX 2 COMPOSITION OF ORAL AND INJECTABLE ESTROGEN–PROGESTOGEN CONTRACEPTIVES Annex 2 lists the composition of brands of estrogen–progestogen preparations used in combined injectables (Table 1), combined oral (Table 2) and phasic oral (Table 3) contraceptives. The countries in which these formulations are used are noted. Are listed only those brands for which availability was reported. The source of these tables is the International Planned Parenthood Foundation (IPPF). Data have been taken from the IPPF 2002 website [http://contraceptive.ippf.org] at the time of the monograph meeting (June 2005). This online site is regularly updated. Table 1. Combined injectables Brand name Composition Countries of availability Acefil Dihydroxyprogesterone acetophenide 150 mg Paraguay + estradiol enanthate 10 mg Agurin Dihydroxyprogesterone acetophenide 150 mg Chile + estradiol enanthate 10 mg Anafertin Dihydroxyprogesterone acetophenide 75 mg El Salvador, Mexico + estradiol enanthate 5 mg Ciclofem Medroxyprogesterone acetate 25 mg Guatemala + estradiol cypionate 5 mg Ciclofemina Medroxyprogesterone acetate 25 mg El Salvador + estradiol cypionate 5 mg Ciclomes Dihydroxyprogesterone acetophenide 150 mg Paraguay + estradiol enanthate 10 mg Ciclovular Dihydroxyprogesterone acetophenide 150 mg Brazil + estradiol enanthate 10 mg Clinomin Dihydroxyprogesterone acetophenide 150 mg Paraguay + estradiol enanthate 10 mg Cyclofem Medroxyprogesterone acetate 25 mg Chile, Indonesia, Malaysia, Mexico, + estradiol cypionate 5 mg Panama, Zimbabwe Cyclofemina Medroxyprogesterone -

Vaccine Purchase Data and Cover Note for Countries
MI4A Vaccine Purchase Data for Countries The MI4A Vaccine Purchase How can my country use this data?1 Database What data is available? • • • • WORKING DOCUMENT - MI4A Vaccine Purchase Data Note [December 2020] | 1 Immunization Agenda IA 2030 Reporting Vaccine Purchase Data Documenting use of MI4A data WORKING DOCUMENT - MI4A Vaccine Purchase Data Note [December 2020] | 2 Key messages to countries • • • 7 WORKING DOCUMENT - MI4A Vaccine Purchase Data Note [December 2020] | 3 • • • • • . / WORKING DOCUMENT - MI4A Vaccine Purchase Data Note [December 2020] | 4 • • • • • • • 12 WORKING DOCUMENT - MI4A Vaccine Purchase Data Note [December 2020] | 5 • • • • • • • • 13 WORKING DOCUMENT - MI4A Vaccine Purchase Data Note [December 2020] | 6 • • • • • WORKING DOCUMENT - MI4A Vaccine Purchase Data Note [December 2020] | 7 What is MI4A? • • • WORKING DOCUMENT - MI4A Vaccine Purchase Data Note [December 2020] | 8 A. Available Products List - as known to WHO The MI4A Products List provides a snapshot overview of vaccine products available for procurement – irrespective of their WHO prequalification status – as of November 2020. This list reflects information reported by countries through the 2020 Joint Reporting Form (JRF) and is supplemented with available information gathered through separate consultations. As part of the MI4A project, WHO conducts in-depth global market studies where the list of available vaccines can be considered comprehensive and up to date. Studies conducted so far include BCG, D&T-containing, measles-containing, meningococcal, HPV, pneumococcal, rabies, and typhoid vaccines. For all the other vaccines it should be noted that this list may not be complete and may be missing some products whose availability is not documented through public reported data and sources. -

Biotechnology in Russia: Why Is It Not a Success Story?
Biotechnology in Russia: Why is it not a success story? Biotechnology inRussia:Whyisitnotasuccessstory? ROGER ROFFEY Biotechnology in Russia: Why is it not a success story? According to President Medvedev 2009 ‘By and large, our industry continues to make the same outdated products and, as a rule, imported generics from substances bought abroad. There is practically no work to create original medicines and technologies.’…‘We must begin the modernisation and technological upgrading of our entire industrial sector. I see this as a question of our country’s survival in the modern world’… ‘These are the key tasks for placing Russia on a new technological level and making it a global leader’. Many states including Russia see biotechnology and its commercialization as a key driver for their future growth. The biotechnology area is characterized by being a very knowledge-intensive activity where there is increasing global competition for know-how. Russia had a very good historical base of R&D and know- how in biotechnology from the previous Soviet military programme. There have been many attempts since 2000 to revive the Russian biotechnology industry not least in 2005 but without much success. In 2009 there were again very ambitious programmes and strategies developed as well as techno-parks for the ROGERROFFEY development of the biotechnology and pharmaceuticals industry up to 2020. It has also been announced by President Medvedev the creation of a Russian equivalent to ‘Silicon Valley’ to include R&D also in biotechnology outside Moscow. There have been many such grand plans but so far they have not been very successful and the question for this study was if it would be different this time? Why are scientists still leaving Russia and foreign investors still hesitating to invest in Russian biotechnology or pharmaceuticals? Why is Russia still not able to compete on the global biotechnology market and is ranked only as number 70 in the world? The current problems and prospects for the biotechnology and pharmaceuticals industries are analysed. -

(ACIP) General Best Guidance for Immunization
Appendix 1: Glossary Adverse event. An undesirable medical condition that occurs following vaccination which might be truly caused by a vaccine, or it might be pure coincidence. Adverse reaction. An undesirable medical condition that has been demonstrated to be caused by a vaccine. Evidence for the causal relation is usually obtained through randomized clinical trials, controlled epidemiologic studies, isolation of the vaccine strain from the pathogenic site, or recurrence of the condition with repeated vaccination (i.e., rechallenge); synonyms include side effect and adverse effect. Adjuvant. A vaccine component distinct from the antigen that enhances the immune response to the antigen. Antitoxin. A solution of antibodies against a toxin. Antitoxin can be derived from either human (e.g., tetanus immune globulin) or animal (usually equine) sources (e.g., diphtheria and botulism antitoxin). Antitoxins are used to confer passive immunity and for treatment. Hyperimmune globulin (specific). Special preparations obtained from blood plasma from donor pools preselected for a high antibody content against a specific antigen (e.g., hepatitis B immune globulin, varicella-zoster immune globulin, rabies immune globulin, tetanus immune globulin, vaccinia immune globulin, cytomegalovirus immune globulin, botulism immune globulin). Immune globulin. A sterile solution containing antibodies, which are usually obtained from human blood. It is obtained by cold ethanol fractionation of large pools of blood plasma and contains 15%-18% protein. Intended for intramuscular administration, immune globulin is primarily indicated for routine maintenance of immunity among certain immunodeficient persons and for passive protection against measles and hepatitis A. General Best Practice Guidelines for Immunization: Appendix 1: Glossary 189 Immunobiologic. Antigenic substances (e.g., vaccines and toxoids) or antibody- containing preparations (e.g., globulins and antitoxins) from human or animal donors. -
![PR-Bird Flu-CCX-[MASTER]](https://docslib.b-cdn.net/cover/9961/pr-bird-flu-ccx-master-1449961.webp)
PR-Bird Flu-CCX-[MASTER]
Avian Flu D-125TM H5N1 It's not a matter of if... it's a matter of WHEN BELIEVED BY US EPA TO KILL AVIAN FLU D-125 is a US EPA-registered sanitizer, disinfectant and cleaner effective against several strains of the Avian Flu. The US EPA does not currently have any products that are regis- tered against the particular H5N1 strain of Avian Flu; however, the US EPA has indicated that they believe D-125 will prove effective against H5N1 once testing is completed. These products are available in an economical concentrate and ready-to-use spray and wipe formulation. Both the concentrate and the ready-to-use are US EPA-registered to kill an extraor- dinary range of 120 plus microbial organisms including all of the pan-demic human influenza strains of the 20th century including: Influenza A / Brazil Virus Influenza A / Victoria (H3N2) Virus Influenza A2-Asian Virus Influenza B Virus (Allen Strain) Influenza C Virus (Taylor Strain) D-125 is a registered tademark of Microgen Inc. Parainfluenza Type 1 In addition to being among a select group of products believed effective against H5N1 Avian Flu Virus, D-125 registered for use in and has specific instructions for disinfecting poultry houses as well as meat, poultry and food processing plants. Microgen Inc. D-125 is also registered for use on both hard, non-porous Clinton Square Executive Center surfaces and on porous surfaces. West Caldwell, NJ 07006 USA www.microgeninc.com With such versatility and efficacy D-125 is the ultimate choice 1-800-420-7522 or 973-575-9025 when disinfecting and sanitizing. -

Live Attenuated Influenza Vaccines Against
ccines & a V f V a o c l c i a n n a Larionova et al., J Vaccines Vaccin 2013, 4:8 r t u i o o n DOI: 10.4172/2157-7560.1000208 J Journal of Vaccines & Vaccination ISSN: 2157-7560 Research Article Open Access Live Attenuated Influenza Vaccines against Highly Pathogenic H5N1avian Influenza: Development and Preclinical Characterization Natalie Larionova1*, Irina Kiseleva1, Irina Isakova-Sivak1, Andrey Rekstin1, Irina Dubrovina1, Ekaterina Bazhenova1, Ted M Ross2, David Swayne3, Larisa Gubareva4, Vadim Tsvetnitsky5, Ekaterina Fedorova1, Elena Doroshenko1 and Larisa Rudenko1 1Institute of Experimental Medicine, St. Petersburg, Russia 2University of Pittsburgh, Pittsburgh, PA, USA 3Southeast Poultry Research Laboratory, Athens, GA, USA 4Centers for Disease Control and Prevention, Atlanta, GA, USA 5PATH (Program for Appropriate Technologies in Health), USA Abstract In this paper we describe the development and the outcomes of the preclinical studies of temperature-sensitive and cold-adapted candidates for live attenuated influenza vaccine (LAIV) based on highly pathogenic avian influenza viruses A/H5N1 with pandemic potential. The LAIV candidates were developed by methods of classical re- assortment between H2N2 Master Donor Virus for Russian LAIV and H5N1 viruses generated by reverse genetics for inactivated vaccine. These reverse genetically generated viruses were chosen as a source of H5 hemagglutinin and contained a modified protease cleavage site believed to be associated with high virulence. The progeny of re- assortment had 7:1 gene composition and were characterized by antigen specificity of the HA of the pandemic virus, a high growth rate in chicken embryos and their parameters of temperature sensitivity and cold adaptation confirmed preserved attenuation of the Master Donor Virus. -

Diphtheria. In: Epidemiology and Prevention of Vaccine
Diphtheria Anna M. Acosta, MD; Pedro L. Moro, MD, MPH; Susan Hariri, PhD; and Tejpratap S.P. Tiwari, MD Diphtheria is an acute, bacterial disease caused by toxin- producing strains of Corynebacterium diphtheriae. The name Diphtheria of the disease is derived from the Greek diphthera, meaning ● Described by Hippocrates in ‘leather hide.’ The disease was described in the 5th century 5th century BCE BCE by Hippocrates, and epidemics were described in the ● Epidemics described in 6th century AD by Aetius. The bacterium was first observed 6th century in diphtheritic membranes by Edwin Klebs in 1883 and cultivated by Friedrich Löffler in 1884. Beginning in the early ● Bacterium first observed in 1900s, prophylaxis was attempted with combinations of toxin 1883 and cultivated in 1884 and antitoxin. Diphtheria toxoid was developed in the early ● Diphtheria toxoid developed 7 1920s but was not widely used until the early 1930s. It was in 1920s incorporated with tetanus toxoid and pertussis vaccine and became routinely used in the 1940s. Corynebacterium diphtheria Corynebacterium diphtheriae ● Aerobic gram-positive bacillus C. diphtheriae is an aerobic, gram-positive bacillus. ● Toxin production occurs Toxin production (toxigenicity) occurs only when the when bacillus is infected bacillus is itself infected (lysogenized) by specific viruses by corynebacteriophages (corynebacteriophages) carrying the genetic information for carrying tox gene the toxin (tox gene). Diphtheria toxin causes the local and systemic manifestations of diphtheria. ● Four biotypes: gravis, intermedius, mitis, and belfanti C. diphtheriae has four biotypes: gravis, intermedius, mitis, ● All isolates should be tested and belfanti. All biotypes can become toxigenic and cause for toxigenicity severe disease.