Pseudoeurycea Robertsi[I]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aquiloeurycea Scandens (Walker, 1955). the Tamaulipan False Brook Salamander Is Endemic to Mexico
Aquiloeurycea scandens (Walker, 1955). The Tamaulipan False Brook Salamander is endemic to Mexico. Originally described from caves in the Reserva de la Biósfera El Cielo in southwestern Tamaulipas, this species later was reported from a locality in San Luis Potosí (Johnson et al., 1978) and another in Coahuila (Lemos-Espinal and Smith, 2007). Frost (2015) noted, however, that specimens from areas remote from the type locality might be unnamed species. This individual was found in an ecotone of cloud forest and pine-oak forest near Ejido La Gloria, in the municipality of Gómez Farías. Wilson et al. (2013b) determined its EVS as 17, placing it in the middle portion of the high vulnerability category. Its conservation status has been assessed as Vulnerable by IUCN, and as a species of special protection by SEMARNAT. ' © Elí García-Padilla 42 www.mesoamericanherpetology.com www.eaglemountainpublishing.com The herpetofauna of Tamaulipas, Mexico: composition, distribution, and conservation status SERGIO A. TERÁN-JUÁREZ1, ELÍ GARCÍA-PADILLA2, VICENTE Mata-SILva3, JERRY D. JOHNSON3, AND LARRY DavID WILSON4 1División de Estudios de Posgrado e Investigación, Instituto Tecnológico de Ciudad Victoria, Boulevard Emilio Portes Gil No. 1301 Pte. Apartado postal 175, 87010, Ciudad Victoria, Tamaulipas, Mexico. Email: [email protected] 2Oaxaca de Juárez, Oaxaca, Código Postal 68023, Mexico. E-mail: [email protected] 3Department of Biological Sciences, The University of Texas at El Paso, El Paso, Texas 79968-0500, United States. E-mails: [email protected] and [email protected] 4Centro Zamorano de Biodiversidad, Escuela Agrícola Panamericana Zamorano, Departamento de Francisco Morazán, Honduras. E-mail: [email protected] ABSTRACT: The herpetofauna of Tamaulipas, the northeasternmost state in Mexico, is comprised of 184 species, including 31 anurans, 13 salamanders, one crocodylian, 124 squamates, and 15 turtles. -

Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca
Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or other participating organizations. Published by: IUCN, Gland, Switzerland Copyright: © 2015 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Lamoreux, J. F., McKnight, M. W., and R. Cabrera Hernandez (2015). Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca. Gland, Switzerland: IUCN. xxiv + 320pp. ISBN: 978-2-8317-1717-3 DOI: 10.2305/IUCN.CH.2015.SSC-OP.53.en Cover photographs: Totontepec landscape; new Plectrohyla species, Ixalotriton niger, Concepción Pápalo, Thorius minutissimus, Craugastor pozo (panels, left to right) Back cover photograph: Collecting in Chamula, Chiapas Photo credits: The cover photographs were taken by the authors under grant agreements with the two main project funders: NGS and CEPF. -

Multi-National Conservation of Alligator Lizards
MULTI-NATIONAL CONSERVATION OF ALLIGATOR LIZARDS: APPLIED SOCIOECOLOGICAL LESSONS FROM A FLAGSHIP GROUP by ADAM G. CLAUSE (Under the Direction of John Maerz) ABSTRACT The Anthropocene is defined by unprecedented human influence on the biosphere. Integrative conservation recognizes this inextricable coupling of human and natural systems, and mobilizes multiple epistemologies to seek equitable, enduring solutions to complex socioecological issues. Although a central motivation of global conservation practice is to protect at-risk species, such organisms may be the subject of competing social perspectives that can impede robust interventions. Furthermore, imperiled species are often chronically understudied, which prevents the immediate application of data-driven quantitative modeling approaches in conservation decision making. Instead, real-world management goals are regularly prioritized on the basis of expert opinion. Here, I explore how an organismal natural history perspective, when grounded in a critique of established human judgements, can help resolve socioecological conflicts and contextualize perceived threats related to threatened species conservation and policy development. To achieve this, I leverage a multi-national system anchored by a diverse, enigmatic, and often endangered New World clade: alligator lizards. Using a threat analysis and status assessment, I show that one recent petition to list a California alligator lizard, Elgaria panamintina, under the US Endangered Species Act often contradicts the best available science. -

Extreme Morphological and Ecological Homoplasy in Tropical Salamanders
Extreme morphological and ecological homoplasy in tropical salamanders Gabriela Parra-Olea* and David B. Wake† Museum of Vertebrate Zoology and Department of Integrative Biology, University of California, Berkeley, CA 94720-3160 Contributed by David B. Wake, April 25, 2001 Fossorial salamanders typically have elongate and attenuated We analyzed sequences of mtDNA of many tropical bolito- heads and bodies, diminutive limbs, hands and feet, and extremely glossines, including all recognized genera, and determined that elongate tails. Batrachoseps from California, Lineatriton from east- Lineatriton and Oedipina are much more closely related to other ern Me´xico, and Oedipina from southern Me´xico to Ecuador, all taxa than to each other (3, 4). Not only was Lineatriton deeply members of the family Plethodontidae, tribe Bolitoglossini, resem- nested within the large, mainly Mexican genus Pseudoeurycea, ble one another in external morphology, which has evolved inde- but populations of Lineatriton from different parts of its geo- pendently. Whereas Oedipina and Batrachoseps are elongate be- graphic range were more closely related to different species of cause there are more trunk vertebrae, a widespread homoplasy Pseudoeurycea than to each other. Here we analyze molecular (parallelism) in salamanders, the genus Lineatriton is unique in data for 1,816 bp of mtDNA derived from three genes, reject the having evolved convergently by an alternate ‘‘giraffe-neck’’ de- monophyly of Lineatriton, and support an extraordinary case of velopmental program. Lineatriton has the same number of trunk homoplasy in a putative species that previously has been con- vertebrae as related, nonelongated taxa, but individual trunk sidered to be extremely specialized, and unique, in both mor- vertebrae are elongated. -
Comparative Osteology and Evolution of the Lungless Salamanders, Family Plethodontidae David B
COMPARATIVE OSTEOLOGY AND EVOLUTION OF THE LUNGLESS SALAMANDERS, FAMILY PLETHODONTIDAE DAVID B. WAKE1 ABSTRACT: Lungless salamanders of the family Plethodontidae comprise the largest and most diverse group of tailed amphibians. An evolutionary morphological approach has been employed to elucidate evolutionary rela tionships, patterns and trends within the family. Comparative osteology has been emphasized and skeletons of all twenty-three genera and three-fourths of the one hundred eighty-three species have been studied. A detailed osteological analysis includes consideration of the evolution of each element as well as the functional unit of which it is a part. Functional and developmental aspects are stressed. A new classification is suggested, based on osteological and other char acters. The subfamily Desmognathinae includes the genera Desmognathus, Leurognathus, and Phaeognathus. Members of the subfamily Plethodontinae are placed in three tribes. The tribe Hemidactyliini includes the genera Gyri nophilus, Pseudotriton, Stereochilus, Eurycea, Typhlomolge, and Hemidac tylium. The genera Plethodon, Aneides, and Ensatina comprise the tribe Pleth odontini. The highly diversified tribe Bolitoglossini includes three super genera. The supergenera Hydromantes and Batrachoseps include the nominal genera only. The supergenus Bolitoglossa includes Bolitoglossa, Oedipina, Pseudoeurycea, Chiropterotriton, Parvimolge, Lineatriton, and Thorius. Manculus is considered to be congeneric with Eurycea, and Magnadig ita is congeneric with Bolitoglossa. Two species are assigned to Typhlomolge, which is recognized as a genus distinct from Eurycea. No. new information is available concerning Haptoglossa. Recognition of a family Desmognathidae is rejected. All genera are defined and suprageneric groupings are defined and char acterized. Range maps are presented for all genera. Relationships of all genera are discussed. -

Rediscovery at the Type Locality and Detection of a New Population 1José L
Offcial journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(2) [General Section]: 126–132 (e193). Thorius narismagnus (Amphibia: Plethodontidae): rediscovery at the type locality and detection of a new population 1José L. Aguilar-López, 2,*Paulina García-Bañuelos, 3Eduardo Pineda, and 4Sean M. Rovito 1,2,3Red de Biología y Conservación de Vertebrados, Instituto de Ecología, A.C., Carretera antigua a Coatepec 351, El Haya, Xalapa, Veracruz, MEXICO 4Unidad de Genómica Avanzada (Langebio), Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, km 9.6 Libramiento Norte Carretera Irapuato-León, Irapuato, Guanajuato CP 36824, MEXICO Abstract.—Of the 42 Critically Endangered species of plethodontid salamanders that occur in Mexico, thirteen have not been reported in more than ten years. Given the lack of reports since 1976, the minute plethodontid salamander Thorius narismagnus is widely considered as missing. However, this report describes the rediscovery of this minute salamander at the type locality (Volcán San Martín), as well as a new locality on Volcán Santa Marta, 28 km southeast of its previously known distribution, both in the Los Tuxtlas region of Veracruz, Mexico. The localities where T. narismagnus has been found are mature forests in a community reserve on Volcán San Martín and a private reserve on Volcán Santa Marta. The presence of maxillary teeth, generally absent in Thorius, are reported here in some T. narismagnus females. Two efforts which may contribute to the conservation of Thorius narismagnus are the preservation of the cloud forests where this species persists, as well as the determination of the presence and possible effect of chytrid fungus in these populations. -

Pseudoeurycea Naucampatepetl. the Cofre De Perote Salamander Is Endemic to the Sierra Madre Oriental of Eastern Mexico. This
Pseudoeurycea naucampatepetl. The Cofre de Perote salamander is endemic to the Sierra Madre Oriental of eastern Mexico. This relatively large salamander (reported to attain a total length of 150 mm) is recorded only from, “a narrow ridge extending east from Cofre de Perote and terminating [on] a small peak (Cerro Volcancillo) at the type locality,” in central Veracruz, at elevations from 2,500 to 3,000 m (Amphibian Species of the World website). Pseudoeurycea naucampatepetl has been assigned to the P. bellii complex of the P. bellii group (Raffaëlli 2007) and is considered most closely related to P. gigantea, a species endemic to the La specimens and has not been seen for 20 years, despite thorough surveys in 2003 and 2004 (EDGE; www.edgeofexistence.org), and thus it might be extinct. The habitat at the type locality (pine-oak forest with abundant bunch grass) lies within Lower Montane Wet Forest (Wilson and Johnson 2010; IUCN Red List website [accessed 21 April 2013]). The known specimens were “found beneath the surface of roadside banks” (www.edgeofexistence.org) along the road to Las Lajas Microwave Station, 15 kilometers (by road) south of Highway 140 from Las Vigas, Veracruz (Amphibian Species of the World website). This species is terrestrial and presumed to reproduce by direct development. Pseudoeurycea naucampatepetl is placed as number 89 in the top 100 Evolutionarily Distinct and Globally Endangered amphib- ians (EDGE; www.edgeofexistence.org). We calculated this animal’s EVS as 17, which is in the middle of the high vulnerability category (see text for explanation), and its IUCN status has been assessed as Critically Endangered. -

Assessing Environmental Variables Across Plethodontid Salamanders
Lauren Mellenthin1, Erica Baken1, Dr. Dean Adams1 1Iowa State University, Ames, Iowa Stereochilus marginatus Pseudotriton ruber Pseudotriton montanus Gyrinophilus subterraneus Gyrinophilus porphyriticus Gyrinophilus palleucus Gyrinophilus gulolineatus Urspelerpes brucei Eurycea tynerensis Eurycea spelaea Eurycea multiplicata Introduction Eurycea waterlooensis Eurycea rathbuni Materials & Methods Eurycea sosorum Eurycea tridentifera Eurycea pterophila Eurycea neotenes Eurycea nana Eurycea troglodytes Eurycea latitans Eurycea naufragia • Arboreality has evolved at least 5 times within Plethodontid salamanders. [1] Eurycea tonkawae Eurycea chisholmensis Climate Variables & Eurycea quadridigitata Polygons & Point Data Eurycea wallacei Eurycea lucifuga Eurycea longicauda Eurycea guttolineata MAXENT Modeling Eurycea bislineata Eurycea wilderae Eurycea cirrigera • Yet no morphological differences separate arboreal and terrestrial species. [1] Eurycea junaluska Hemidactylium scutatum Batrachoseps robustus Batrachoseps wrighti Batrachoseps campi Batrachoseps attenuatus Batrachoseps pacificus Batrachoseps major Batrachoseps luciae Batrachoseps minor Batrachoseps incognitus • There is minimal range overlap between the two microhabitat types. Batrachoseps gavilanensis Batrachoseps gabrieli Batrachoseps stebbinsi Batrachoseps relictus Batrachoseps simatus Batrachoseps nigriventris Batrachoseps gregarius Preliminary results discovered that 71% of the arboreal species distribution Batrachoseps diabolicus Batrachoseps regius Batrachoseps kawia Dendrotriton -

Amphibia: Caudata: Plethodontidae
AMPHIBIA: CAUDATA: PLETHODONTIDAE Catalogue of American Amphibian and Reptiles. Parra-Olea, G. 1998. Pseudoeutyea nigrornnculntn. Pseudoeurycea nigromaculata Taylor Boliroglossa nigromac~ilnraTaylor 194 1 : 14 1. Type locality, "Cuautlapan, Veracruz [I g052'N, 97'0 1 'W; Mexico]." Holotype, National Museum of Natural History (USNM) 110635, adult female. collected January-February 1940 by H.M. Smith (not examined by author). Psa~rcloeu~cennigrornaculntn Taylor 1944:209. CONTENT. No subspecies are recognized. DEFINITION. Adult Pserrrloenpceo nigromnculatn are ro- bust and of moderate size, with mean SVL = 49.2mm (44.9- 55.8). Females tend to be larger than males, but no significant difference in total body length occurs. Tail length is longer than SVL (2-12 mm longer). Costal grooves number 13. The limbs are long and, when adpressed, are separated by a space of 1-2.5 costal grooves. The digits are long and webbed at their bases. Toes are broadly flattened and tips are truncate. Vomerine teeth number 35 (mean) and about 60 maxillary teeth are present. In alcohol a pattern of different intensities of brown is present along the body. The neck is dark brown, the head and dorsum are lighter, and the tail is light brown or beige. Distinct scat- tered black spots are present all along the dorsum, on the flanks, and all around the tail. The density of spots is higher on the head. The underside of the head and the venter are uniformly MAP. Distribution of Pseudoelrrycea nigromocrrlrrrrr. The circle marks medium brown and without spots. Color in life was described the type locality and the dot indicates the only other known locality. -

50 CFR Ch. I (10–1–20 Edition) § 16.14
§ 15.41 50 CFR Ch. I (10–1–20 Edition) Species Common name Serinus canaria ............................................................. Common Canary. 1 Note: Permits are still required for this species under part 17 of this chapter. (b) Non-captive-bred species. The list 16.14 Importation of live or dead amphib- in this paragraph includes species of ians or their eggs. non-captive-bred exotic birds and coun- 16.15 Importation of live reptiles or their tries for which importation into the eggs. United States is not prohibited by sec- Subpart C—Permits tion 15.11. The species are grouped tax- onomically by order, and may only be 16.22 Injurious wildlife permits. imported from the approved country, except as provided under a permit Subpart D—Additional Exemptions issued pursuant to subpart C of this 16.32 Importation by Federal agencies. part. 16.33 Importation of natural-history speci- [59 FR 62262, Dec. 2, 1994, as amended at 61 mens. FR 2093, Jan. 24, 1996; 82 FR 16540, Apr. 5, AUTHORITY: 18 U.S.C. 42. 2017] SOURCE: 39 FR 1169, Jan. 4, 1974, unless oth- erwise noted. Subpart E—Qualifying Facilities Breeding Exotic Birds in Captivity Subpart A—Introduction § 15.41 Criteria for including facilities as qualifying for imports. [Re- § 16.1 Purpose of regulations. served] The regulations contained in this part implement the Lacey Act (18 § 15.42 List of foreign qualifying breed- U.S.C. 42). ing facilities. [Reserved] § 16.2 Scope of regulations. Subpart F—List of Prohibited Spe- The provisions of this part are in ad- cies Not Listed in the Appen- dition to, and are not in lieu of, other dices to the Convention regulations of this subchapter B which may require a permit or prescribe addi- § 15.51 Criteria for including species tional restrictions or conditions for the and countries in the prohibited list. -

Functional Morphology and Evolution of Tail Autotomy in Salamanders
Functional Morphology and Evolution of Tail Autotomy in Salamanders DAVID B. WAKE AND IAN G. DRESNER Department of Anatomy, The University of Chicago, Chicago, lllinois ABSTRACT Basal tail constriction occurs in about two-thirds of the species of plethodontid salamanders. The constriction, which marks the site of tail autotomy, is a result of a reduction in length and diameter of the first caudal segment. Gross and microscopic anatomical studies reveal that many structural specializations are associated with basal constriction, and these are considered in detail. Areas of weak- ness in the skin at the posterior end of the first caudal segment, at the attachment of the musculature to the intermyotomal septum at the anterior end of the same segment, and between the last caudosacral and first caudal vertebrae precisely define the route of tail breakage. During autotomy the entire tail is shed, and a cylinder of skin one segment long closes over the wound at the end of the body. It is suggested that specializations described in this paper have evolved jndepend- ently in three different groups of salamanders. Experiments and field observations reveal that, contrary to expectations, frequency of tail breakage is less in species with apparent provisions for tail autotomy than in less specialized species. The tail is a very important, highly functional organ in salamanders and it is suggested that selection has been for behavioral and structural adaptations for control of tail loss, rather than for tail loss per se. Tail loss and subsequent regeneration is sent facts concerning anatomical details a well documented phenomenon among of the basal tail region, a functional inter- the lower vertebrates. -
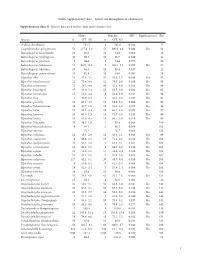
I Online Supplementary Data – Sexual Size Dimorphism in Salamanders
Online Supplementary data – Sexual size dimorphism in salamanders Supplementary data S1. Species data used in this study and references list. Males Females SSD Significant test Ref Species n SVL±SD n SVL±SD Andrias davidianus 2 532.5 8 383.0 -0.280 12 Cryptobranchus alleganiensis 53 277.4±5.2 52 300.9±3.4 0.084 Yes 61 Batrachuperus karlschmidti 10 80.0 10 84.8 0.060 26 Batrachuperus londongensis 20 98.6 10 96.7 -0.019 12 Batrachuperus pinchonii 5 69.6 5 74.6 0.070 26 Batrachuperus taibaiensis 11 92.9±12.1 9 102.1±7.1 0.099 Yes 27 Batrachuperus tibetanus 10 94.5 10 92.8 -0.017 12 Batrachuperus yenyuadensis 10 82.8 10 74.8 -0.096 26 Hynobius abei 24 57.8±2.1 34 55.0±1.2 -0.048 Yes 92 Hynobius amakusaensis 22 75.4±4.8 12 76.5±3.6 0.014 No 93 Hynobius arisanensis 72 54.3±4.8 40 55.2±4.8 0.016 No 94 Hynobius boulengeri 37 83.0±5.4 15 91.5±3.8 0.102 Yes 95 Hynobius formosanus 15 53.0±4.4 8 52.4±3.9 -0.011 No 94 Hynobius fuca 4 50.9±2.8 3 52.8±2.0 0.037 No 94 Hynobius glacialis 12 63.1±4.7 11 58.9±5.2 -0.066 No 94 Hynobius hidamontanus 39 47.7±1.0 15 51.3±1.2 0.075 Yes 96 Hynobius katoi 12 58.4±3.3 10 62.7±1.6 0.073 Yes 97 Hynobius kimurae 20 63.0±1.5 15 72.7±2.0 0.153 Yes 98 Hynobius leechii 70 61.6±4.5 18 66.5±5.9 0.079 Yes 99 Hynobius lichenatus 37 58.5±1.9 2 53.8 -0.080 100 Hynobius maoershanensis 4 86.1 2 80.1 -0.069 101 Hynobius naevius 72.1 76.7 0.063 102 Hynobius nebulosus 14 48.3±2.9 12 50.4±2.1 0.043 Yes 96 Hynobius osumiensis 9 68.4±3.1 15 70.2±3.0 0.026 No 103 Hynobius quelpaertensis 41 52.5±3.8 4 61.3±4.1 0.167 Yes 104 Hynobius