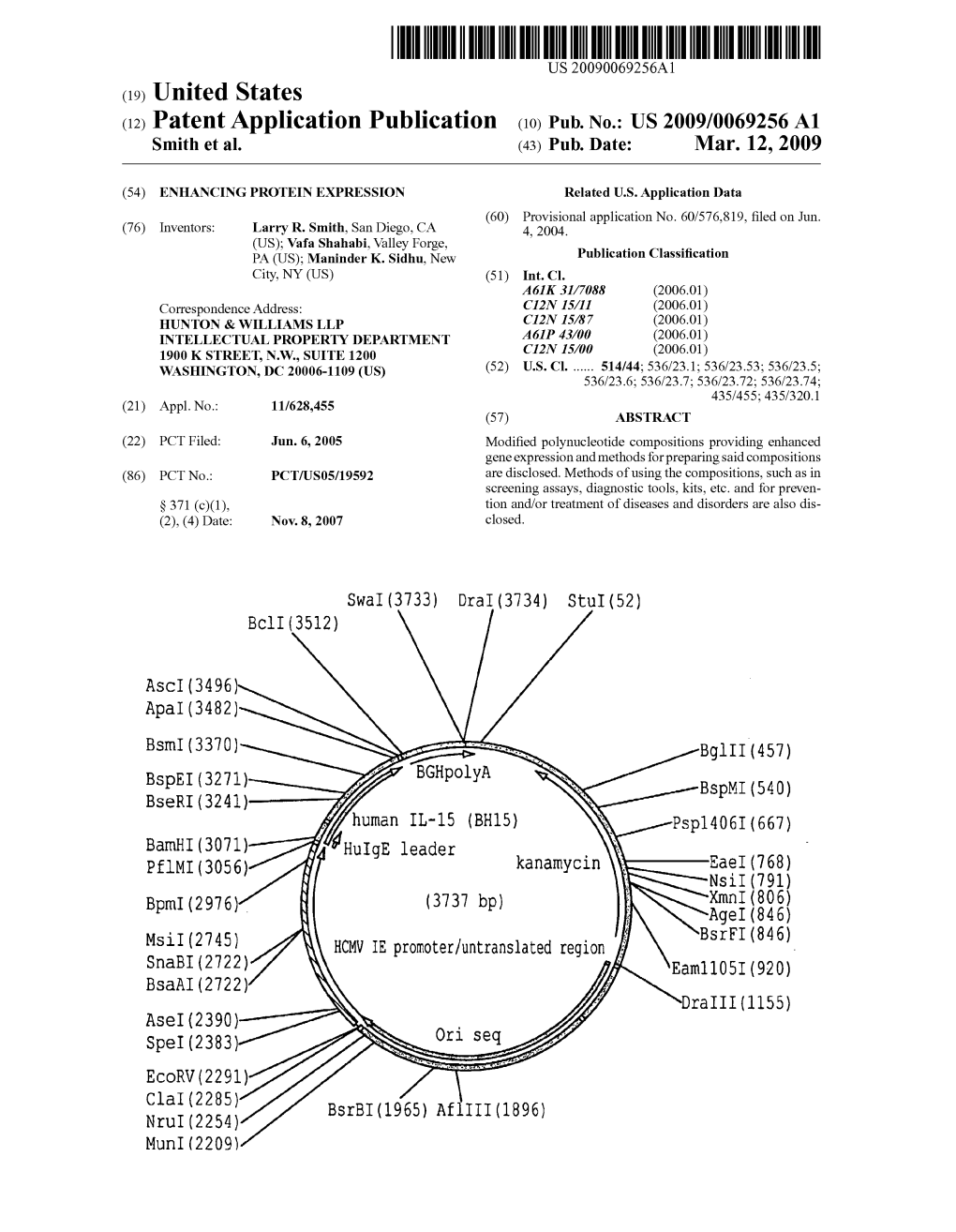
(12) Patent Application Publication (10) Pub. No.: US 2009/0069256A1 Smith Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nucleotide Sequence and Phylogenetic Analysis of a Bamboo Mosaic Potexvirus Isolate from Common Bamboo (Bambusa Vulgaris Mcclure)
YangBot. Bull. et al. Acad. Nucleotide Sin. (1997) sequence 38: 77-84 of BaMV-V RNA 77 Nucleotide sequence and phylogenetic analysis of a bamboo mosaic potexvirus isolate from common bamboo (Bambusa vulgaris McClure) Chi-Chen Yang1,2, Jih-Shiou Liu1, Chan-Pin Lin2 and Na-Sheng Lin1,3 1Institute of Botany, Academia Sinica, Taipei, Taiwan 115, Republic of China 2Department of Plant Pathology and Entomology, National Taiwan University, Taipei, Taiwan, Republic of China (Received September 20, 1996; Accepted January 30, 1997) Abstract. The complete cDNA sequence corresponding to the genomic RNA of an isolate bamboo mosaic potexvirus (BaMV-V) from common bamboo (Bambusa vulgaris McClure) was determined. This isolate is the first potexvirus with which a satellite RNA has been associated. The genome organization of BaMV-V, similar to those of other potexviruses, contained five open reading frames (ORFs 15) coding for polypeptides with molecular weight of 156 kDa, 28 kDa, 13 kDa, 6 kDa, and 25 kDa, respectively. Nucleotide sequence analysis showed a 10.0% difference from the BaMV-O isolate previously described whereas the amino acid comparison showed a difference of 3.2%. When three conservative domains of RNA dependent RNA polymerase (RdRp) were used for phylogenetic analysis, the greatest variation between two strains of each virus was only 12.8% of that between the two closest members of the potexvirus group. The grouping of potexviruses distinct from other groups of plant viruses was also confirmed by a comparision of three conservative motifs of RdRp. Keywords: Bamboo mosaic virus; Nucleotide sequence; Potexvirus. Introduction virus M, PVM) (Zavriev et al., 1991), hordeivirus (e.g. -

Tamada-Text R3 HK-TT
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Okayama University Scientific Achievement Repository Tamada & Kondo - 1 Journal of General Plant Pathology Biological and genetic diversity of plasmodiophorid-transmitted viruses and their vectors Tetsuo Tamada* and Hideki Kondo Institute of Plant Science and Resources (IPSR), Okayama University, Kurashiki 710-0046, Japan. Current address for T. Tamada: Kita 10, Nishi 1, 13-2-606, Sapporo, 001-0010, Japan. *Corresponding author: Tetsuo Tamada; E-mail: [email protected] Total text pages 32 Word counts: 10 words (title); 147 words (Abstract); 6004 words (Main Text) Tables: 3; Figure: 1; Supplementary figure: 1 Tamada & Kondo - 2 Abstract About 20 species of viruses belonging to five genera, Benyvirus, Furovirus, Pecluvirus, Pomovirus and Bymovirus, are known to be transmitted by plasmodiophorids. These viruses have all positive-sense single-stranded RNA genomes that consist of two to five RNA components. Three species of plasmodiophorids are recognized as vectors: Polymyxa graminis, P. betae, and Spongospora subterranea. The viruses can survive in soil within the long-lived resting spores of the vector. There are biological and genetic variations in both virus and vector species. Many of the viruses have become the causal agents of important diseases in major crops, such as rice, wheat, barley, rye, sugar beet, potato, and groundnut. Control measure is dependent on the development of the resistant cultivars. During the last half a century, several virus diseases have been rapidly spread and distributed worldwide. For the six major virus diseases, their geographical distribution, diversity, and genetic resistance are addressed. -

The Development and Characterisation of Grapevine Virus-Based
The development and characterisation of grapevine virus-based expression vectors by Jacques du Preez Presented in partial fulfilment of the requirements for the degree Doctor of Philosophy at the Department of Genetics, Stellenbosch University March 2010 Supervisors: Prof JT Burger and Dr DE Goszczynski Study leader: Dr D Stephan Declaration I, the undersigned, hereby declare that the work contained in this thesis is my own original work and that I have not previously in its entirety or in part submitted it at any university for a degree. ______________________ Date: _______________ Jacques du Preez Copyright © 2010 Stellenbosch University All rights reserved ii Abstract Grapevine ( Vitis vinifera L.) is a very important agricultural commodity that needs to be protected. To achieve this several in vivo tools are needed for the study of this crop and the pathogens that infect it. Recently the grapevine genome has been sequenced and the next important step will be gene annotation and function using these in vivo tools. In this study the use of Grapevine virus A (GVA), genus Vitivirus , family Flexiviridae , as transient expression and VIGS vector for heterologous protein expression and functional genomics in Nicotiana benthamiana and V . vinifera were evaluated. Full-length genomic sequences of three South African variants of the virus (GTR11, GTG111 and GTR12) were generated and used in a molecular sequence comparison study. Results confirmed the separation of GVA variants into three groups, with group III (mild variants) being the most distantly related. It showed the high molecular heterogeneity of the virus and that ORF 2 was the most diverse. The GVA variants GTG111, GTR12 and GTR11 were placed in molecular groups I, II and III respectively. -

Disruption of Virus Movement Confers Broad-Spectrum Resistance Against
Proc. Nati. Acad. Sci. USA Vol. 91, pp. 10310-10314, October 1994 Plant Biology Disruption of virus movement confers broad-spectrum resistance against systemic infection by plant viruses with a triple gene block (trnenic plant/doinat negative mutaton/ n l movement proein) DAVID L. BECK, CRAIG J. VAN DOLLEWEERD, TONY J. LOUGH, EZEQUIEL BALMORI, DAVIN M. VOOT, MARK T. ANDERSEN, IONA E. W. O'BRIEN, AND RICHARD L. S. FORSTERt Molecular Genetics Group, The Horticultural and Food Research Institute of New Zealand Ltd., Private Bag 92169, Auckland, New Zealand Communicated by George Bruening, June 23, 1994 ABSTRACT White clover mosaic virus strain 0 (WCIMV- tially difficult. New forms ofresistance active against several 0), species of the Potexvirus genus, contains a set of three different viruses or groups of viruses are being sought. One partially overlapping genes (the triple gene block) that encodes such approach involved the introduction of the gene coding nonvirion proteins of 26 kDa, 13 kDa, and 7 kDa. These for rat 2'-5' oligoadenylate synthetase into the genome of proteins are necesy for cell-to-cell movement in plants but potato plants (4). Genetically engineering transgenic plants to not for replication. The WCIMV-O 13-kDa gene was mutated block virus movement, mimicking the mechanism of some (to 13*) in a region of the gene that is conserved in all viruses natural resistance genes, has been proposed (1, 5, 6) but known to possess triple-gene-block proteins. All 10 13* trans- remains mostly unexploited. genic lines of Nicodiana benthamiana designed to express the The movement function of plant viruses can be comple- mutated movement protein were shown to be resistant to mented by another, frequently unrelated, virus in double systemic infection by WCIMV-O at 1 jug ofWCIMV virions per infections (7). -
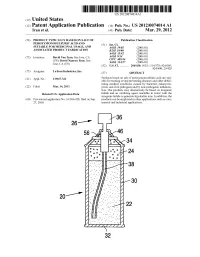
Us 2012/0074.014 A1 2 .. 1
US 2012O074O14A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0074.014 A1 Tran et al. (43) Pub. Date: Mar. 29, 2012 (54) PRODUCT TYPICALLY BASED ON SALT OF Publication Classification PEROXYMONOSULFURIC ACID AND (51) Int. Cl SUITABLE FOR MEDICINAL USAGE, AND iBio/02 (2006.01) ASSOCATED PRODUCT FABRICATION B23P 19/00 (2006.01) A633/42 (2006.01) (75) Inventors: David Van Tran, San Jose, CA A6IR 9/14 (2006.01) (US); David Nguyen Tran, San CD7C 409/244 (2006.01) Jose, CA (US) A6II 3/327 (2006.01) s (52) U.S. Cl. ............. 206/438: 562/1; 514/578; 424/605; 424/400; 29/428 (73) Assignee: LuTran Industries, Inc. (57) ABSTRACT 21) Appl. No.: 13AO47,742 Products based on salt of peroxyperoxVmonosulfuric acid are suit (21) Appl. No 9 able for treating or/and preventing diseases and other debili tating medical conditions caused by bacterial, eukaryotic, (22) Filed: Mar. 14, 2011 prion, and viral pathogens and by non-pathogenic inflamma tion. The products may alternatively be based on inorganic Related U.S. Application Data halide and an oxidizing agent reactable in water with the inorganic halide to generate hypohalite ions. In addition, the (60) Provisional application No. 61/386,928, filed on Sep. products can be employed in other applications such as com 27, 2010. mercial and industrial applications. 5T. 2 .. 2. 1.4. J., Patent Application Publication Mar. 29, 2012 Sheet 1 of 2 US 2012/0074014 A1 cro Q-D 22 Fig. 2a Fig.2b Patent Application Publication Mar. 29, 2012 Sheet 2 of 2 US 2012/0074014 A1 90 92 US 2012/0074014 A1 Mar. -

Progress and Prospects of Studies on Polymyxa Graminis and Its Transmitted Cereal Viruses in China*
PROGRESS IN NATURAL SCIENCE V ol .15 , No .6 , June 2005 REVIEW ARTICLE Progress and prospects of studies on Polymyxa graminis and its transmitted cereal viruses in China* CH EN Jianping ** (Virology and Biotechnology Institute , Zhejiang Academy of Agricultural Sciences, Key Laboratory of Plant Virology , Ministry of Agri- culture and Zhejiang Province, Hangzhou 310021, China) Received October 8 , 2004 ;revised October 25 , 2004 Abstract Polymyxa gram inis is a eukaryotic obligate biotrophic parasite of plant roots that belongs to a poorly studied discrete taxonomic unit informally called “ plasmodiophorids” .P .graminis is nonpathogenic , but has the ability to acquire and transmit nine plant viruses w hich belong to genera Bymovirus and Furovirus and cause serious diseases in cereal crop speciesand also result in significant yield reductions in China and elsew here.Genus Bymovirus contains barley yellow mosaic virus (BaYMV), barley mild mosaic virus (BaMMV), w heat yellow mosaic virus (WYMV), w heat spindle streak mosaic virus (WSS MV), and oat mosaic virus (OMV), and genus F urovirus contains soil-borne w heat mosaic virus(S BW MV), oat golden stripe virus(OGSV), and new ly identified Chinese w heat mosaic virus(CWM V)and soil-borne cereal mosaic virus(S BCMV).All these viruses have been sequenced and their w orldw ide distribu- tions have been studied .The viruses are protected by the environment w ithin P .gra minis resting spores that may remain dormant but vi- able for decades(probably until a suitable host plant is encountered).S pontaneous deletion mutants of S BW MV , OGS V and OMV are de- tected , and these deletion mutants are not transmissible by the fungus.The persistent, soil-borne nature of these diseases makes the use of virus-resistant crop varieties cu rren tly the only practical and environmentally friendly means to control them , and a large number of disease resistant germ plasms have been screened . -

Multipartite Viruses: Organization, Emergence and Evolution
UNIVERSIDAD AUTÓNOMA DE MADRID FACULTAD DE CIENCIAS DEPARTAMENTO DE BIOLOGÍA MOLECULAR MULTIPARTITE VIRUSES: ORGANIZATION, EMERGENCE AND EVOLUTION TESIS DOCTORAL Adriana Lucía Sanz García Madrid, 2019 MULTIPARTITE VIRUSES Organization, emergence and evolution TESIS DOCTORAL Memoria presentada por Adriana Luc´ıa Sanz Garc´ıa Licenciada en Bioqu´ımica por la Universidad Autonoma´ de Madrid Supervisada por Dra. Susanna Manrubia Cuevas Centro Nacional de Biotecnolog´ıa (CSIC) Memoria presentada para optar al grado de Doctor en Biociencias Moleculares Facultad de Ciencias Departamento de Biolog´ıa Molecular Universidad Autonoma´ de Madrid Madrid, 2019 Tesis doctoral Multipartite viruses: Organization, emergence and evolution, 2019, Madrid, Espana. Memoria presentada por Adriana Luc´ıa-Sanz, licenciada en Bioqumica´ y con un master´ en Biof´ısica en la Universidad Autonoma´ de Madrid para optar al grado de doctor en Biociencias Moleculares del departamento de Biolog´ıa Molecular en la facultad de Ciencias de la Universidad Autonoma´ de Madrid Supervisora de tesis: Dr. Susanna Manrubia Cuevas. Investigadora Cient´ıfica en el Centro Nacional de Biotecnolog´ıa (CSIC), C/ Darwin 3, 28049 Madrid, Espana. to the reader CONTENTS Acknowledgments xi Resumen xiii Abstract xv Introduction xvii I.1 What is a virus? xvii I.2 What is a multipartite virus? xix I.3 The multipartite lifecycle xx I.4 Overview of this thesis xxv PART I OBJECTIVES PART II METHODOLOGY 0.5 Database management for constructing the multipartite and segmented datasets 3 0.6 Analytical -

Downloaded by the Reader
Virology Journal BioMed Central Commentary Open Access International Committee on Taxonomy of Viruses and the 3,142 unassigned species CM Fauquet*1 and D Fargette2 Address: 1International Laboratory for Tropical Agricultural Biotechnology, Danforth Plant Science Center, 975 N. Warson Rd., St. Louis, MO 63132 USA and 2Institut de Recherche pour le Developpement, BP 64501, 34394 Montpellier cedex 5, France Email: CM Fauquet* - [email protected]; D Fargette - [email protected] * Corresponding author Published: 16 August 2005 Received: 08 July 2005 Accepted: 16 August 2005 Virology Journal 2005, 2:64 doi:10.1186/1743-422X-2-64 This article is available from: http://www.virologyj.com/content/2/1/64 © 2005 Fauquet and Fargette; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract In 2005, ICTV (International Committee on Taxonomy of Viruses), the official body of the Virology Division of the International Union of Microbiological Societies responsible for naming and classifying viruses, will publish its latest report, the state of the art in virus nomenclature and taxonomy. The book lists more than 6,000 viruses classified in 1,950 species and in more than 391 different higher taxa. However, GenBank contains a staggering additional 3,142 "species" unaccounted for by the ICTV report. This paper reviews the reasons for such a situation and suggests what might be done in the near future to remedy this problem, particularly in light of the potential for a ten-fold increase in virus sequencing in the coming years that would generate many unclassified viruses. -

Molecular Characterization of an Unusual New Plant RNA Virus Reveals an Evolutionary Link Between Two Different Virus Families
RESEARCH ARTICLE Molecular characterization of an unusual new plant RNA virus reveals an evolutionary link between two different virus families 1 1 2 2 Sun-Jung Kwon , Gug-Seoun Choi , Boram Choi , Jang-Kyun SeoID * 1 Horticultural and Herbal Crop Environment Division, National Institute of Horticultural and Herbal Science, Wanju, Republic of Korea, 2 Department of International Agricultural Technology and Institutes of Green Bio Science and Technology, Seoul National University, Pyeongchang, Republic of Korea * [email protected] a1111111111 a1111111111 a1111111111 a1111111111 Abstract a1111111111 An unusual novel plant virus provisionally named goji berry chlorosis virus (GBCV) was iso- lated from goji berry plants (Lycium chinense Miller) showing chlorosis symptoms and its complete genome sequence was determined. The viral genome consists of a positive- sense single-stranded RNA of 10,100 ribonucleotides and contains six open reading frames OPEN ACCESS (ORFs). Electron microscopy showed that the viral genome is packaged as a filamentous Citation: Kwon S-J, Choi G-S, Choi B, Seo J-K particle with an average length of approximately 850 nm. Phylogenetic analysis and amino (2018) Molecular characterization of an unusual acid similarity analysis of the encoded ORFs revealed that this new virus could be classified new plant RNA virus reveals an evolutionary link between two different virus families. PLoS ONE 13 in an intermediate position between the families Benyviridae and Virgaviridae. The GBCV (10): e0206382. https://doi.org/10.1371/journal. 200-kDa replicase (ORF1) is more similar to benyvirus replicases than to virgavirus repli- pone.0206382 cases, while its 17-kDa coat protein (CP, ORF2) is more closely related with virgavirus CPs Editor: A. -

Donkey Orchid Symptomless Virus: a Viral 'Platypus'
Donkey Orchid Symptomless Virus: A Viral ‘Platypus’ from Australian Terrestrial Orchids Stephen J. Wylie*, Hua Li, Michael G. K. Jones Australian Plant Virology Laboratory, Western Australian State Agricultural Biotechnology Centre, School of Veterinary and Life Sciences, Murdoch University, Perth, Australia Abstract Complete and partial genome sequences of two isolates of an unusual new plant virus, designated Donkey orchid symptomless virus (DOSV) were identified using a high-throughput sequencing approach. The virus was identified from asymptomatic plants of Australian terrestrial orchid Diuris longifolia (Common donkey orchid) growing in a remnant forest patch near Perth, western Australia. DOSV was identified from two D. longifolia plants of 264 tested, and from at least one plant of 129 Caladenia latifolia (pink fairy orchid) plants tested. Phylogenetic analysis of the genome revealed open reading frames (ORF) encoding seven putative proteins of apparently disparate origins. A 69- kDa protein (ORF1) that overlapped the replicase shared low identity with MPs of plant tymoviruses (Tymoviridae). A 157-kDa replicase (ORF2) and 22-kDa coat protein (ORF4) shared 32% and 40% amino acid identity, respectively, with homologous proteins encoded by members of the plant virus family Alphaflexiviridae. A 44-kDa protein (ORF3) shared low identity with myosin and an autophagy protein from Squirrelpox virus. A 27-kDa protein (ORF5) shared no identity with described proteins. A 14-kDa protein (ORF6) shared limited sequence identity (26%) over a limited region of the envelope glycoprotein precursor of mammal-infecting Crimea-Congo hemorrhagic fever virus (Bunyaviridae). The putative 25-kDa movement protein (MP) (ORF7) shared limited (27%) identity with 3A-like MPs of members of the plant-infecting Tombusviridae and Virgaviridae. -

Rothamsted Repository Download
Patron: Her Majesty The Queen Rothamsted Research Harpenden, Herts, AL5 2JQ Telephone: +44 (0)1582 763133 WeB: http://www.rothamsted.ac.uk/ Rothamsted Repository Download A - Papers appearing in refereed journals Diao, A., Chen, J., Gitton, F., Antoniw, J. F., Mullins, J., Hall, A. M. and Adams, M. J. 1999. Sequences of European wheat mosaic virus and oat golden stripe virus and genome analysis of the genus Furovirus. Virology. 261 (2), pp. 331-339. The publisher's version can be accessed at: • https://dx.doi.org/10.1006/viro.1999.9880 The output can be accessed at: https://repository.rothamsted.ac.uk/item/8817z/sequences-of-european-wheat-mosaic- virus-and-oat-golden-stripe-virus-and-genome-analysis-of-the-genus-furovirus. © 1 September 1999, Academic Press Inc Elsevier Science. 25/10/2019 15:03 repository.rothamsted.ac.uk [email protected] Rothamsted Research is a Company Limited by Guarantee Registered Office: as above. Registered in England No. 2393175. Registered Charity No. 802038. VAT No. 197 4201 51. Founded in 1843 by John Bennet Lawes. Virology 261, 331–339 (1999) Article ID viro.1999.9880, available online at http://www.idealibrary.com on Sequences of European Wheat Mosaic Virus and Oat Golden Stripe Virus and Genome Analysis of the Genus Furovirus Aipo Diao,*,† Jianping Chen,† Francine Gitton,*,‡ John F. Antoniw,* Jonathan Mullins,§ Avice M. Hall,¶ and Michael J. Adams*,1 *Plant Pathology Department, IACR-Rothamsted, Harpenden, Herts AL5 2JQ, United Kingdom; †Virology Laboratory, Zhejiang Academy of Agricultural Sciences, -

A Furoviral Replicase Recruits Host HSP70 to Membranes for Viral RNA
www.nature.com/scientificreports OPEN A furoviral replicase recruits host HSP70 to membranes for viral RNA replication Received: 09 January 2017 Jian Yang1, Fen Zhang1,2, Nian-Jun Cai1,2, Ne Wu1,3, Xuan Chen1,3, Jing Li1, Xiang-Feng Meng4, Accepted: 01 March 2017 Tong-Quan Zhu4, Jian-Ping Chen1 & Heng-Mu Zhang1,2 Published: 03 April 2017 Many host factors have been identified to be involved in viral infection. However, although furoviruses cause important diseases of cereals worldwide, no host factors have yet been identified that interact with furoviral genes or participate in the viral infection cycle. In this study, both TaHSP70 and NbHSP70 were up-regulated in Chinese wheat mosaic furovirus (CWMV)-infected plants. Their overexpression and inhibition were correlated with the accumulation of viral genomic RNAs, suggesting that the HSP70 genes could be necessary for CWMV infection. The subcellular distributions of TaHSP70 and NbHSP70 were significantly affected by CWMV infection or by infiltration of RNA1 alone. Further assays showed that the viral replicase encoded by CWMV RNA1 interacts with both TaHSP70 and NbHSP70 in vivo and vitro and that its region aa167–333 was responsible for the interaction. Subcellular assays showed that the viral replicase could recruit both TaHSP70 and NbHSP70 from the cytoplasm or nucleus to the granular aggregations or inclusion-like structures on the intracellular membrane system, suggesting that both HSP70s may be recruited into the viral replication complex (VRC) to promote furoviral replication. This is the first host factor identified to be involved in furoviral infection, which extends the list and functional scope of HSP70 chaperones.