Transduction by Bacteriophage Ti * Henry Drexler
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mobile Genetic Elements in Streptococci
Curr. Issues Mol. Biol. (2019) 32: 123-166. DOI: https://dx.doi.org/10.21775/cimb.032.123 Mobile Genetic Elements in Streptococci Miao Lu#, Tao Gong#, Anqi Zhang, Boyu Tang, Jiamin Chen, Zhong Zhang, Yuqing Li*, Xuedong Zhou* State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, PR China. #Miao Lu and Tao Gong contributed equally to this work. *Address correspondence to: [email protected], [email protected] Abstract Streptococci are a group of Gram-positive bacteria belonging to the family Streptococcaceae, which are responsible of multiple diseases. Some of these species can cause invasive infection that may result in life-threatening illness. Moreover, antibiotic-resistant bacteria are considerably increasing, thus imposing a global consideration. One of the main causes of this resistance is the horizontal gene transfer (HGT), associated to gene transfer agents including transposons, integrons, plasmids and bacteriophages. These agents, which are called mobile genetic elements (MGEs), encode proteins able to mediate DNA movements. This review briefly describes MGEs in streptococci, focusing on their structure and properties related to HGT and antibiotic resistance. caister.com/cimb 123 Curr. Issues Mol. Biol. (2019) Vol. 32 Mobile Genetic Elements Lu et al Introduction Streptococci are a group of Gram-positive bacteria widely distributed across human and animals. Unlike the Staphylococcus species, streptococci are catalase negative and are subclassified into the three subspecies alpha, beta and gamma according to the partial, complete or absent hemolysis induced, respectively. The beta hemolytic streptococci species are further classified by the cell wall carbohydrate composition (Lancefield, 1933) and according to human diseases in Lancefield groups A, B, C and G. -

Horizontal Gene Transfer
Genetic Variation: The genetic substrate for natural selection Horizontal Gene Transfer Dr. Carol E. Lee, University of Wisconsin Copyright ©2020; Do not upload without permission What about organisms that do not have sexual reproduction? In prokaryotes: Horizontal gene transfer (HGT): Also termed Lateral Gene Transfer - the lateral transmission of genes between individual cells, either directly or indirectly. Could include transformation, transduction, and conjugation. This transfer of genes between organisms occurs in a manner distinct from the vertical transmission of genes from parent to offspring via sexual reproduction. These mechanisms not only generate new gene assortments, they also help move genes throughout populations and from species to species. HGT has been shown to be an important factor in the evolution of many organisms. From some basic background on prokaryotic genome architecture Smaller Population Size • Differences in genome architecture (noncoding, nonfunctional) (regulatory sequence) (transcribed sequence) General Principles • Most conserved feature of Prokaryotes is the operon • Gene Order: Prokaryotic gene order is not conserved (aside from order within the operon), whereas in Eukaryotes gene order tends to be conserved across taxa • Intron-exon genomic organization: The distinctive feature of eukaryotic genomes that sharply separates them from prokaryotic genomes is the presence of spliceosomal introns that interrupt protein-coding genes Small vs. Large Genomes 1. Compact, relatively small genomes of viruses, archaea, bacteria (typically, <10Mb), and many unicellular eukaryotes (typically, <20 Mb). In these genomes, protein-coding and RNA-coding sequences occupy most of the genomic sequence. 2. Expansive, large genomes of multicellular and some unicellular eukaryotes (typically, >100 Mb). In these genomes, the majority of the nucleotide sequence is non-coding. -
Recombination in Bacteria
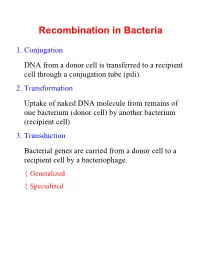
Recombination in Bacteria 1. Conjugation DNA from a donor cell is transferred to a recipient cell through a conjugation tube (pili). 2. Transformation Uptake of naked DNA molecule from remains of one bacterium (donor cell) by another bacterium (recipient cell). 3. Transduction Bacterial genes are carried from a donor cell to a recipient cell by a bacteriophage. { Generalized { Specialized Conjugation Ability to conjugate located on the F-plasmid F+ Cells act as donors F- Cells act as recipients F+/F- Conjugation: { F Factor “replicates off” a single strand of DNA. { New strand goes through pili to recipient cell. { New strand is made double stranded. { If entire F-plasmid crosses, then recipient cell becomes F+, otherwise nothing happens Conjugation with Hfr Hfr cell (High Frequency Recombination) cells have F-plasmid integrated into the Chromosome. Integration into the Chromosome is unique for each F-plasmid strain. When F-plasmid material is replicated and sent across pili, Chromosomal material is included. (Figure 6.10 in Klug & Cummings) When chromosomal material is in recipient cell, recombination can occur: { Recombination is double stranded. { Donor genes are recombined into the recipient cell. { Corresponding genes from recipient cell are recombined out of the chromosome and reabsorbed by the cell. Interrupted Mating Mapping 1. Allow conjugation to start Genes closest to the origin of replication site (in the direction of replication) are moved through the pili first. 2. After a set time, interrupt conjugation Only those genes closest to the origin of replication site will conjugate. The long the time, the more that is able to conjugate. 3. -

Microbial Genetics by Dr Preeti Bajpai
Dr. Preeti Bajpai Genes: an overview ▪ A gene is the functional unit of heredity ▪ Each chromosome carry a linear array of multiple genes ▪ Each gene represents segment of DNA responsible for synthesis of RNA or protein product ▪ A gene is considered to be unit of genetic information that controls specific aspect of phenotype DNA Chromosome Gene Protein-1 Prokaryotic Courtesy: Team Shrub https://twitter.com/realscientists/status/927 cell 667237145767937 Genetic exchange within Prokaryotes The genetic exchange occurring in bacteria involve transfers of genes from one bacterium to another. The gene transfer in prokaryotic cells is thus unidirectional and the recombination events usually occur between a fragment of one chromosome (from a donor cell) and a complete chromosome (in a recipient cell) Mechanisms for genetic exchange Bacteria exchange genetic material through three different parasexual processes* namely transformation, conjugation and transduction. *Parasexual process involves recombination of genes from genetically distinct cells occurring without involvement of meiosis and fertilization Principles of Genetics-sixth edition Courtesy: Beatrice the Biologist.com by D. Peter Snustad & Michael J. Simmons (http://www.beatricebiologist.com/2014/08/bacterial-gifts/) Transformation: an introduction Transformation involves the uptake of free DNA molecules released from one bacterium (the donor cell) by another bacterium (the recipient cell). Frederick Griffith discovered transformation in Streptococcus pneumoniae (pneumococcus) in 1928. In his experiments, Griffith used two related strains of bacteria, known as R and S. The R bacteria (nonvirulent) formed colonies, or clumps of related bacteria, that Frederick Griffith 1877-1941 had a rough appearance (hence the abbreviation "R"). The S bacteria (virulent) formed colonies that were rounded and smooth (hence the abbreviation "S"). -

Comprehensive Analysis of Mobile Genetic Elements in the Gut Microbiome Reveals Phylum-Level Niche-Adaptive Gene Pools
bioRxiv preprint doi: https://doi.org/10.1101/214213; this version posted December 22, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Comprehensive analysis of mobile genetic elements in the gut microbiome 2 reveals phylum-level niche-adaptive gene pools 3 Xiaofang Jiang1,2,†, Andrew Brantley Hall2,3,†, Ramnik J. Xavier1,2,3,4, and Eric Alm1,2,5,* 4 1 Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, 5 Cambridge, MA 02139, USA 6 2 Broad Institute of MIT and Harvard, Cambridge, MA 02142, USA 7 3 Center for Computational and Integrative Biology, Massachusetts General Hospital and Harvard 8 Medical School, Boston, MA 02114, USA 9 4 Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General 10 Hospital and Harvard Medical School, Boston, MA 02114, USA 11 5 MIT Department of Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA 12 02142, USA 13 † Co-first Authors 14 * Corresponding Author bioRxiv preprint doi: https://doi.org/10.1101/214213; this version posted December 22, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 15 Abstract 16 Mobile genetic elements (MGEs) drive extensive horizontal transfer in the gut microbiome. This transfer 17 could benefit human health by conferring new metabolic capabilities to commensal microbes, or it could 18 threaten human health by spreading antibiotic resistance genes to pathogens. Despite their biological 19 importance and medical relevance, MGEs from the gut microbiome have not been systematically 20 characterized. -

Molecular Model for the Transposition and Replication of Bacteriophage
Proc. Natl. Acad. Sci. USA Vol. 76, No. 4, pp. 1933-1937, April 1979 Genetics Molecular model for the transposition and replication of bacteriophage Mu and other transposable elements (DNA insertion elements/nonhomologous recombination/site-specific recombination/replicon fusion/topoisomerases) JAMES A. SHAPIRO Department of Microbiology, The University of Chicago, Chicago, Illinois 60637 Communicated by Hewson Swift, December 11, 1978 ABSTRACT A series of molecular events will explain how B genetic elements can transpose from one DNA site to another, B y generate a short oligonucleotide duplication at both ends of the I % new insertion site, and replicate in the transposition process. I These events include the formation of recombinant molecules A a% /; C which have been postulated to be intermediates in the trans- position process. The model explains how the replication of bacteriophage Mu is obligatorily associated with movement to z x x new genetic sites. It postulates that all transposable elements replicate in the transposition process so that they remain at their z original site while moving to new sites. According to this model, the mechanism of transposition is very different from the in- V sertion and excision of bacteriophage X. y FIG. 1. Replicon fusion. The boxes with an arrow indicate copies Recent research on transposable elements in bacteria has pro- of a transposable element. The letters mark arbitrary regions of the vided important insights into the role of nonhomologous re- two replicons to indicate the relative positions of the elements in the combination in genetic rearrangements (1-4). These elements donor and cointegrate molecules. include small insertion sequences (IS elements), transposable resistance determinants (Tn elements), and bacteriophage Mu results in the duplication of a short oligonucleotide sequence (3). -

Section 4. Guidance Document on Horizontal Gene Transfer Between Bacteria
306 - PART 2. DOCUMENTS ON MICRO-ORGANISMS Section 4. Guidance document on horizontal gene transfer between bacteria 1. Introduction Horizontal gene transfer (HGT) 1 refers to the stable transfer of genetic material from one organism to another without reproduction. The significance of horizontal gene transfer was first recognised when evidence was found for ‘infectious heredity’ of multiple antibiotic resistance to pathogens (Watanabe, 1963). The assumed importance of HGT has changed several times (Doolittle et al., 2003) but there is general agreement now that HGT is a major, if not the dominant, force in bacterial evolution. Massive gene exchanges in completely sequenced genomes were discovered by deviant composition, anomalous phylogenetic distribution, great similarity of genes from distantly related species, and incongruent phylogenetic trees (Ochman et al., 2000; Koonin et al., 2001; Jain et al., 2002; Doolittle et al., 2003; Kurland et al., 2003; Philippe and Douady, 2003). There is also much evidence now for HGT by mobile genetic elements (MGEs) being an ongoing process that plays a primary role in the ecological adaptation of prokaryotes. Well documented is the example of the dissemination of antibiotic resistance genes by HGT that allowed bacterial populations to rapidly adapt to a strong selective pressure by agronomically and medically used antibiotics (Tschäpe, 1994; Witte, 1998; Mazel and Davies, 1999). MGEs shape bacterial genomes, promote intra-species variability and distribute genes between distantly related bacterial genera. Horizontal gene transfer (HGT) between bacteria is driven by three major processes: transformation (the uptake of free DNA), transduction (gene transfer mediated by bacteriophages) and conjugation (gene transfer by means of plasmids or conjugative and integrated elements). -

Molecular Biology and Applied Genetics
MOLECULAR BIOLOGY AND APPLIED GENETICS FOR Medical Laboratory Technology Students Upgraded Lecture Note Series Mohammed Awole Adem Jimma University MOLECULAR BIOLOGY AND APPLIED GENETICS For Medical Laboratory Technician Students Lecture Note Series Mohammed Awole Adem Upgraded - 2006 In collaboration with The Carter Center (EPHTI) and The Federal Democratic Republic of Ethiopia Ministry of Education and Ministry of Health Jimma University PREFACE The problem faced today in the learning and teaching of Applied Genetics and Molecular Biology for laboratory technologists in universities, colleges andhealth institutions primarily from the unavailability of textbooks that focus on the needs of Ethiopian students. This lecture note has been prepared with the primary aim of alleviating the problems encountered in the teaching of Medical Applied Genetics and Molecular Biology course and in minimizing discrepancies prevailing among the different teaching and training health institutions. It can also be used in teaching any introductory course on medical Applied Genetics and Molecular Biology and as a reference material. This lecture note is specifically designed for medical laboratory technologists, and includes only those areas of molecular cell biology and Applied Genetics relevant to degree-level understanding of modern laboratory technology. Since genetics is prerequisite course to molecular biology, the lecture note starts with Genetics i followed by Molecular Biology. It provides students with molecular background to enable them to understand and critically analyze recent advances in laboratory sciences. Finally, it contains a glossary, which summarizes important terminologies used in the text. Each chapter begins by specific learning objectives and at the end of each chapter review questions are also included. -

The Art of Transfection (Poster / Pdf)
TRANSDUCTION NON-VIRAL TRANSFECTION Transduction is the process of using vectors including retroviruses, lentiviruses, adenoviruses, PACKAGE DELIVERY: Chemical Chemical transfection adeno-associated viruses, or hybrids to deliver genetic payloads into cells. Generally, a plasmid transfection reagent containing mRNA carrying genes flanked by viral sequences is first transfected into a producer cell with other reagent virus-associated (packaging) plasmids. In the producer cells, virions form that contain the gene The Art of Transfection of interest. For safety, no plasmid used in the process contains all of the necessary sequences Inserting genetic material into mammalian and insect cells without killing them can be a challenge, CHEMICAL TRANSFECTION for virion formation, and only the plasmid carrying the gene of interest contains signals that but scientists have developed several ways to perform this intricate task. Transfection is the process of Functional proteins or allow it to be packaged into virions. Researchers then extract, purify, and use the virions from Complexation structural components released Chemical carriers represent the most straightforward and widespread tools for gene delivery the producer cells to insert DNA into other cells to stably or transiently express the DNA of introducing nucleic acids (plasmid DNA or messenger, short interfering, or micro RNA) into a cell. from cell or into cytoplasm experiments in mammalian cells. Chemical transfection experiments follow a simple workflow and interest. The transferred genetic material, which lacks viral genes, cannot generate new viruses. Researchers accomplish this with nonviral methods (chemical or physical transfection), or with viral provide high efficiency nucleic acid delivery for the most commonly used cells as well as many methods, commonly referred to as transduction. -

Transducing Units (TU) Determination 4
1. Bioinformatic and Gene expression 2. Viral based vectors 3. In vitro cell assays Transducing units (TU) determination 4. In vivo models Vectalys is an R&D company with a state-of-the-art technology platform for customized viral vector production. The company has developed a unique process enabling the production of high titer high purity lentiviral vectors for optimal knock-down or over expression in relevant models: primary and stem cells for target gene validation and specific tissue for animal models. Method of titration Titers of lentiviral vectors and wild type viruses critically depend on the method of titration used. The titer can be estimated by measuring the number of efficient viral particles or transducing units. In this case, the titer obtained only includes those particles which are able to transduce cells. > from cell binding to viral DNA integration, the titer corresponds to the number of integrated genomes and is quantified by qPCR. > from cell binding to protein expression, the titer corresponds to the number of transducing units and is quantified by FACS. This is a functional titer. Titers determined by qPCR are normalized with our standard GFP expressing vector titrated by FACS. So, all our qPCR or FACS titers are strictly comparable for any transgene. 1 2 3 4 5 Integrated qPCR genomes 6 1. Receptor recognition 2. Entry 7 3. Reverse transcription 4. Nuclear translocation Expression 5. Integration 8 FACS 6. Transcription of GFP 7. Splicing 8. Translation Both quantifications are performed after the transduction of cells and critically depend on cell and vector characteristics. > First, the cells must be readily permissive to all steps of viral transduction. -

Genetic Exchange in Bacteria
Systems Microbiology Monday Oct 16 - Ch 10 -Brock Genetic Exchange in Bacteria •• HomologousHomologous recombinationrecombination •• TransformationTransformation •• PlasmidsPlasmids andand conjugationconjugation •• TransposableTransposable elementselements •• TransductionTransduction (virus(virus mediatedmediated xchangexchange)) Gene exchange in bacteria • Transfer of DNA from one bacterium to another is a common means of gene dispersal. It has a big effect on bacterial evolution, and tremendous practical implications. For example, lateral transfer is responsible for the spread drug resistance determinants between bacterial species. • Three common mechanisms of lateral gene exchange : – Transformation (extracellular DNA uptake) – Conjugation (bacterial mating systems) – Transduction (viral mediated gene exchange) RecA mediated Homologous recombination Images removed due to copyright restrictions. See Figures 10-9 and 10-10 in Madigan, Michael, and John Martinko. Brock Biology of Microorganisms.11th ed. Upper Saddle River, NJ: Pearson Prentice Hall, 2006. ISBN: 0131443291. Gene exchange in bacteriaTransformation The Griffith Experiment S Injection Dead mouse; • Discovered by Griffith in yields S1 cells 1928 during the course of his Live "smooth" (encapsulated) studies of virulence in type 1 pneumococci (S1) Streptococcus pneumoniae. Dead S Live mouse Heat-killed S1 • S=smooth colony Live mouse morphotype Live "rough" (unencapsulated) pneumococci (R1 or R2) derived by subculture from S1 or S2, respectively • R=rough colony Dead mouse; yields S cells morphotype R1 + Dead S + 1 Live R1 Killed S1 R2 + Dead S + Dead mouse; yields S1 cells Live R2 Killed S2 Figure by MIT OCW. Gene exchange mechanisms in bacteria Transformation The Griffith Experiment Injection Dead mouse; S yields S cells Avery, MacLeod, and McCarthy (1944) 1 fractionation studies led to conclusion that Live "smooth" (encapsulated) type 1 pneumococci (S ) transformation principle is DNA. -

Ability to Ferment Galactose) to Gal- Tmutants of Escherichia Coli K12 Has Been Described in a Previous Report (MORSE, LEDERBERG,And LEDERBERG1956)
TRANSDUCTIONAL HETEROGENOTES IN ESCHERICHIA COLI' M. L. MORSE: ESTHER M. LEDERBERG, AND JOSHUA LEDERBERG Department of Genetics, University of Wisconsin, Madison Received April 12, 1956 HE transduction of the Gal+ factor (ability to ferment galactose) to Gal- Tmutants of Escherichia coli K12 has been described in a previous report (MORSE, LEDERBERG,and LEDERBERG1956). The galactose positive transduction clones were often found to be unstable and to throw off Gal- types about once per thousand divisions. We postulated that the transformed cells were heterogenotic (heterozygous for the transduced fragment), and that the instability was a result of segregation. This process has been studied in more detail with several non-allelic Gal- mutants. Since transduction genetics is a system analogous but not identical with sexual crossing, which also occurs in these strains, a distinctive terminology is a useful tool for integrating hypothesis and experiment. The following definitions are given for reference at this point. Their applications will be amplified in the experimental report. GLOSSARY AND SYMBOLS Genetic transduction-transfer of a genetic fragment from one cell to another. Exogenote-a chromosome fragment; usually relates to the donor in transduction. Endogenote-homologous part of the intact chromosome which corresponds to a given exogenote; usually relates to the recipient in transduction. Syngenote-(cf. synkaryon) a cell whose genetic complement includes an exo- genote (i.e. is hyperploid for a fragment). Heterogenote-(cf. heterozygote) a syngenote in which the exogenote and endo- genote differ in one or more markers. Homogenote-(cf. homozygote) a syngenote in which the exogenote and endo- genote carry the same marker.