Mobile Antimicrobial Resistance Genes in Probiotics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mobile Genetic Elements in Streptococci
Curr. Issues Mol. Biol. (2019) 32: 123-166. DOI: https://dx.doi.org/10.21775/cimb.032.123 Mobile Genetic Elements in Streptococci Miao Lu#, Tao Gong#, Anqi Zhang, Boyu Tang, Jiamin Chen, Zhong Zhang, Yuqing Li*, Xuedong Zhou* State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, PR China. #Miao Lu and Tao Gong contributed equally to this work. *Address correspondence to: [email protected], [email protected] Abstract Streptococci are a group of Gram-positive bacteria belonging to the family Streptococcaceae, which are responsible of multiple diseases. Some of these species can cause invasive infection that may result in life-threatening illness. Moreover, antibiotic-resistant bacteria are considerably increasing, thus imposing a global consideration. One of the main causes of this resistance is the horizontal gene transfer (HGT), associated to gene transfer agents including transposons, integrons, plasmids and bacteriophages. These agents, which are called mobile genetic elements (MGEs), encode proteins able to mediate DNA movements. This review briefly describes MGEs in streptococci, focusing on their structure and properties related to HGT and antibiotic resistance. caister.com/cimb 123 Curr. Issues Mol. Biol. (2019) Vol. 32 Mobile Genetic Elements Lu et al Introduction Streptococci are a group of Gram-positive bacteria widely distributed across human and animals. Unlike the Staphylococcus species, streptococci are catalase negative and are subclassified into the three subspecies alpha, beta and gamma according to the partial, complete or absent hemolysis induced, respectively. The beta hemolytic streptococci species are further classified by the cell wall carbohydrate composition (Lancefield, 1933) and according to human diseases in Lancefield groups A, B, C and G. -

Exploring Antibiotic Susceptibility, Resistome and Mobilome Structure of Planctomycetes from Gemmataceae Family
sustainability Article Exploring Antibiotic Susceptibility, Resistome and Mobilome Structure of Planctomycetes from Gemmataceae Family Anastasia A. Ivanova *, Kirill K. Miroshnikov and Igor Y. Oshkin Research Center of Biotechnology of the Russian Academy of Sciences, Winogradsky Institute of Microbiology, 119071 Moscow, Russia; [email protected] (K.K.M.); [email protected] (I.Y.O.) * Correspondence: [email protected] Abstract: The family Gemmataceae accomodates aerobic, chemoorganotrophic planctomycetes with large genome sizes, is mostly distributed in freshwater and terrestrial environments. However, these bacteria have recently also been found in locations relevant to human health. Since the antimi- crobial resistance genes (AMR) from environmental resistome have the potential to be transferred to pathogens, it is essential to explore the resistant capabilities of environmental bacteria. In this study, the reconstruction of in silico resistome was performed for all nine available gemmata genomes. Furthermore, the genome of the newly isolated yet-undescribed strain G18 was sequenced and added to all analyses steps. Selected genomes were screened for the presence of mobile genetic elements. The flanking location of mobilizable genomic milieu around the AMR genes was of particular in- terest since such colocalization may appear to promote the horizontal gene transfer (HGT) events. Moreover the antibiotic susceptibility profile of six phylogenetically distinct strains of Gemmataceae planctomycetes was determined. Citation: Ivanova, A.A.; Keywords: planctomycetes; Gemmataceae; antibiotic resistance profile; resistome; mobilome Miroshnikov, K.K.; Oshkin, I.Y. Exploring Antibiotic Susceptibility, Resistome and Mobilome Structure of Planctomycetes from Gemmataceae 1. Introduction Family. Sustainability 2021, 13, 5031. Several decades ago humanity faced the global issue of growing antibiotic resistance https://doi.org/10.3390/su13095031 of bacterial pathogens in clinic [1–5]. -

Bifidobacterium Response to Lactulose Ingestion in the Gut Relies on A
ARTICLE https://doi.org/10.1038/s42003-021-02072-7 OPEN Bifidobacterium response to lactulose ingestion in the gut relies on a solute-binding protein- dependent ABC transporter ✉ Keisuke Yoshida 1 , Rika Hirano2,3, Yohei Sakai4, Moonhak Choi5, Mikiyasu Sakanaka 5,6, Shin Kurihara3,6, Hisakazu Iino7, Jin-zhong Xiao 1, Takane Katayama5,6 & Toshitaka Odamaki1 This study aims to understand the mechanistic basis underlying the response of Bifido- bacterium to lactulose ingestion in guts of healthy Japanese subjects, with specific focus on a lactulose transporter. An in vitro assay using mutant strains of Bifidobacterium longum subsp. 1234567890():,; longum 105-A shows that a solute-binding protein with locus tag number BL105A_0502 (termed LT-SBP) is primarily involved in lactulose uptake. By quantifying faecal abundance of LT-SBP orthologues, which is defined by phylogenetic analysis, we find that subjects with 107 to 109 copies of the genes per gram of faeces before lactulose ingestion show a marked increase in Bifidobacterium after ingestion, suggesting the presence of thresholds between responders and non-responders to lactulose. These results help predict the prebiotics- responder and non-responder status and provide an insight into clinical interventions that test the efficacy of prebiotics. 1 Next Generation Science Institute, RD Division, Morinaga Milk Industry Co., Ltd., Zama, Japan. 2 Research Institute for Bioresources and Biotechnology, Ishikawa Prefectural University, Nonoichi, Japan. 3 Biology-Oriented Science and Technology, Kindai University, Kinokawa, Japan. 4 Food Ingredients and Technology Institute, RD Division, Morinaga Milk Industry Co., Ltd, Zama, Japan. 5 Graduate School of Biostudies, Kyoto University, Kyoto, Japan. 6 Faculty of Bioresources and Environmental Sciences, Ishikawa Prefectural University, Nonoichi, Japan. -

Recursos Naturales - Referencias Bibliográficas
Recursos Naturales - Referencias Bibliográficas Nombre Nombre # Referencia Común Científico Afsar Shaik; Rupesh S Kanhere; Rajaram Cuddapah; Nelson Kumar S; Artemisa, Artemisia Prasanth Reddy Vara; Saisaran Sibyala. Antifertility activity of Artemisia 1 Mugwort vulgaris L. vulgaris leaves on female Wistar rats. Chinese Journal of Natural Medicines 2014, 12(3): 0180-0185 Diandra Araújo Luz, Alana Miranda Pinheiro; Mallone Lopes Silva; Marta Chagas Monteiro; Rui Daniel Prediger; Cristiane Socorro Ferraz Maia; Petiveria 2 Anamú Enéas Andrade Fontes-Júnior. Ethnobotany, phytochemistry and alliacea L. neuropharmacological effects of Petiveria alliacea L. (Phytolaccaceae): A review. Journal of Ethnopharmacology 185 (2016) 182–201 Plantago B. Vanaclocha; S. Cañigueral. Fitoterapia Vademécum de Prescripción. 4° 3 Llantén major L. Edición. p334-335 Turnera B. Vanaclocha; S. Cañigueral. Fitoterapia Vademécum de Prescripción. 3° 4 Damiana diffusa Wild. Edición. p178-179. var. Aesculus Castaño de B. Vanaclocha; S. Cañigueral. Fitoterapia Vademécum de Prescripción. 3° 5 hippocastanu Indias Edición. p172-175 m 1.- Chung-Hua Hsu & Col. The Mushroom Agaricus blazei Murill Extract Normalizes Liver Function in Patients with Chronic Hepatitis B. The Journal of Alternative and Complementary Medicine. Volume 14, Number 3, 2008, pp. 299–301 2.- Diogo G. Valadares, Mariana C. Duarte, Laura Ramírez, Miguel A. Chávez-Fumagalli, Eduardo A.F. Coelho. Prophylactic or therapeutic administration of Agaricus blazei Murill is effective in treatment of murine visceral leishmaniasis. Experimental Parasitology, Volume 132, Issue 2, October 2012, Pages 228-236 6 Setas Agaricus blazei 3.- Chung-Hua Hsu & Col. The Mushroom Agaricus blazei Murill in Combination with Metformin and Gliclazide Improves Insulin Resistance in Type 2 Diabetes: A Randomized, Double-Blinded, and Placebo- Controlled Clinical Trial. -

Delivery of Metabolically Neuroactive Probiotics to the Human Gut
International Journal of Molecular Sciences Article Delivery of Metabolically Neuroactive Probiotics to the Human Gut Peter A. Bron 1, Marta Catalayud 2 , Massimo Marzorati 2,3, Marco Pane 3, Ece Kartal 4 , Raja Dhir 1 and Gregor Reid 5,6,* 1 Seed Health, 2100 Abbot Kinney Blvd Suite G Venice, Los Angeles, CA 90291, USA; [email protected] (P.A.B.); [email protected] (R.D.) 2 ProDigest BV, Technologiepark-Zwijnaarde 94, 9052 Gent, Belgium; [email protected] (M.C.); [email protected] (M.M.) 3 Probioticial, Via Enrico Mattei 3, 28100 Novara, Italy; [email protected] 4 Faculty of Medicine and Heidelberg University Hospital, Institute of Computational Biomedicine, Heidelberg University, Im Neuenheimer Feld 672, 69120 Heidelberg, Germany; [email protected] 5 Centre for Human Microbiome and Probiotic Research, Lawson Health Research Institute, 268 Grosvenor Street, London, ON N6A 4V2, Canada 6 Departments of Microbiology and Immunology, and Surgery, Western University, London, ON N6A 3K7, Canada * Correspondence: [email protected] Abstract: The human microbiome is a rich factory for metabolite production and emerging data has led to the concept that orally administered microbial strains can synthesize metabolites with neuroactive potential. Recent research from ex vivo and murine models suggests translational potential for microbes to regulate anxiety and depression through the gut-brain axis. However, so far, less emphasis has been placed on the selection of specific microbial strains known to produce the required key metabolites and the formulation in which microbial compositions are delivered to the Citation: Bron, P.A.; Catalayud, M.; gut. Here, we describe a double-capsule technology to deliver high numbers of metabolically active Marzorati, M.; Pane, M.; Kartal, E.; Dhir, R.; Reid, G. -

The Resistome of Common Human Pathogens
bioRxiv preprint doi: https://doi.org/10.1101/140194; this version posted May 25, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. The resistome of common human pathogens Christian Munck, Mostafa M. Hashim Ellabaan, Michael Schantz Klausen, Morten O.A. Sommer* The Novo Nordisk Foundation Center for Biosustainability Technical University of Denmark Kogle Alle 6, 2970 Hørsholm, Denmark *[email protected] bioRxiv preprint doi: https://doi.org/10.1101/140194; this version posted May 25, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract Genes capable of conferring resistance to clinically used antibiotics have been found in many different natural environments. However, a concise overview of the resistance genes found in common human bacterial pathogens is lacking, which complicates risk ranking of environmental reservoirs. Here, we present an analysis of potential antibiotic resistance genes in the 17 most common bacterial pathogens isolated from humans. We analyzed more than 20,000 bacterial genomes and defined a clinical resistome as the set of resistance genes found across these genomes. Using this database, we uncovered the co-occurrence frequencies of the resistance gene clusters within each species enabling identification of co-dissemination and co-selection patterns. -

Horizontal Gene Transfer
Genetic Variation: The genetic substrate for natural selection Horizontal Gene Transfer Dr. Carol E. Lee, University of Wisconsin Copyright ©2020; Do not upload without permission What about organisms that do not have sexual reproduction? In prokaryotes: Horizontal gene transfer (HGT): Also termed Lateral Gene Transfer - the lateral transmission of genes between individual cells, either directly or indirectly. Could include transformation, transduction, and conjugation. This transfer of genes between organisms occurs in a manner distinct from the vertical transmission of genes from parent to offspring via sexual reproduction. These mechanisms not only generate new gene assortments, they also help move genes throughout populations and from species to species. HGT has been shown to be an important factor in the evolution of many organisms. From some basic background on prokaryotic genome architecture Smaller Population Size • Differences in genome architecture (noncoding, nonfunctional) (regulatory sequence) (transcribed sequence) General Principles • Most conserved feature of Prokaryotes is the operon • Gene Order: Prokaryotic gene order is not conserved (aside from order within the operon), whereas in Eukaryotes gene order tends to be conserved across taxa • Intron-exon genomic organization: The distinctive feature of eukaryotic genomes that sharply separates them from prokaryotic genomes is the presence of spliceosomal introns that interrupt protein-coding genes Small vs. Large Genomes 1. Compact, relatively small genomes of viruses, archaea, bacteria (typically, <10Mb), and many unicellular eukaryotes (typically, <20 Mb). In these genomes, protein-coding and RNA-coding sequences occupy most of the genomic sequence. 2. Expansive, large genomes of multicellular and some unicellular eukaryotes (typically, >100 Mb). In these genomes, the majority of the nucleotide sequence is non-coding. -

2007151117.Full.Pdf
Spatial and morphological reorganization of endosymbiosis during metamorphosis accommodates adult metabolic requirements in a weevil Justin Mairea,1, Nicolas Parisota, Mariana Galvao Ferrarinia, Agnès Valliera, Benjamin Gilletb, Sandrine Hughesb, Séverine Balmanda, Carole Vincent-Monégata, Anna Zaidman-Rémya,2, and Abdelaziz Heddia,2 aUMR0203, Biologie Fonctionnelle, Insectes et Interactions (BF2i), Institut National des Sciences Appliquées de Lyon (INSA-Lyon), Institut National de Recherche pour l’Agriculture, l’Alimentation et l’Environnement (INRAE), Université de Lyon (Univ Lyon), F-69621 Villeurbanne, France; and bUMR5242, Institut de Génomique Fonctionnelle de Lyon (IGFL), Ecole Normale Supérieure de Lyon, Centre National de la Recherche Scientifique (CNRS), Université Claude Bernard Lyon 1 (UCBL), Université de Lyon (Univ Lyon), F-69007 Lyon, France Edited by John R. Pringle, Stanford University Medical Center, Stanford, CA, and approved June 25, 2020 (received for review April 15, 2020) Bacterial intracellular symbiosis (endosymbiosis) is widespread in allows adaptive decoupling: for example, task specialization of nature and impacts many biological processes. In holometabolous growth at the larval stage versus reproduction at the adult stage symbiotic insects, metamorphosis entails a complete and abrupt (9, 10). However, metamorphosis is also associated with con- internal reorganization that creates a constraint for endosymbiont straints, including higher susceptibility to predators and patho- transmission from larvae to adults. To assess how endosymbiosis gens, as well as the conservation and adaptation of beneficial copes—and potentially evolves—throughout this major host-tissue symbionts (9, 11). In hemimetabolous insects, the relative mor- reorganization, we used the association between the cereal weevil phological stability associated with incomplete metamorphosis is Sitophilus oryzae and the bacterium Sodalis pierantonius as a model believed to facilitate symbiont maintenance and transmission system. -

Transduction by Bacteriophage Ti * Henry Drexler
Proceedings of the National Academy of Sciences Vol. 66, No. 4, pp. 1083-1088, August 1970 Transduction by Bacteriophage Ti * Henry Drexler DEPARTMENT OF MICROBIOLOGY, BOWMAN GRAY SCHOOL OF MEDICINE, WAKE FOREST UNIVERSITY, WINSTON-SALEM, NORTH CAROLINA Communicated by Edward L. Tatum, May 25, 1970 Abstract. Amber mutants of the virulent coliphage T1 are able to transduce a wide variety of genetic characteristics from permissive to nonpermissive K strains of Escherichia coli. The virulent coliphage T1 is not known to be related to any temperate phage. Certain of the characteristics of T1 seem to be incompatible with its potential existence as a temperate phage; for example, the average latent period for T1 is only 13 min' and about 70% of its DNA is derived from the host.2 In order to demonstrate transduction by T1 it is necessray to provide condi- tions in which potential transductants are able to survive; this is accomplished by using amber mutants of T1 to transduce nonpermissive recipients. Experi- ments which show that T1 is a transducing phage are presented and have been designed chiefly to illustrate the following points: (1) Infection by T1 is able to cause heritable changes in recipients; (2) the genotype of the donor host is im- portant in determining what characteristics can be transferred to recipients; (3) the changes in the recipients are not caused by transformation; and (4) there is a similarity between the transducing activity and the plaque-forming ability of T1 with respect to serology, host range, and density. Materials and Methods. Bacterial strains: The abbreviations and symbols of Demerec et al.3 and Taylor and Trotter4 are used to describe all pertinent genotypes. -

Our Probiotic Strains Str Ou Ainsr Or Pr S Tobioic
THERAPEUTIC IndEx GAStroenteroloGy pag. 5 OUROUR PROBIOTICPROOBIOTTIC ImmunoloGy pag. 17 DermAtoloGy pag. 21 STRAINSSTRRAINSS AntI-AGInG pag. 23 CArDIoloGy/ metAbolISm pag. 27 DIetetICS pag. 29 GIneColoGy pag. 31 uroloGy pag. 39 Gut-brAIn pag. 43 Probiotics like nobody else OurOur InnovativeInnovative orAl CAre pag. 47 Probiotical S.p.A. Via E. Mattei, 3, 28100 Novara (NO), Italy - T: +39 0321 46 59 33 61 Strains:Sttrains:i F: +39 0321 49 26 93 - email: [email protected] - www.probiotical.com teChnoloGIeS pag. 49 ReadyReaddy forfor YourYour HealthHeallth tHeraPeUtiC index GastroenteroloGy pag. 5 ImmunoloGy pag. 17 DermatoloGy pag. 20 antI-aGInG pag. 22 CarDIoloGy/metabolIsm pag. 25 DIetetICs pag. 27 GIneColoGy pag. 29 uroloGy pag. 37 Gut-braIn pag. 41 oral Care pag. 44 teChnoloGIes pag. 45 Romane Catalogo Indice 13-5-20:Layout 1 26/05/20 17:44 Pagina 1 Probiotical is driving probiotics innovation since 1985. Our expertise comprises strain selection, advanced R&D, production of strains to finished products with all guarantees of stability and demonstrated efficacy in many functionalities. Our strains can be proposed in allergen-free quality, microencapsulated, and standardized in different concentrations. Strains available as raw material can be provided in bulk or as customized finished products. Strains available as finished dosage form can be provided only as Probiotical finished products. Romane Catalogo Indice 13-5-20:Layout 1 26/05/20 17:44 Pagina 3 StrainS & blendS index Bifidobacterium • Bifidobacterium breve - BR03™ (DSM 16604) Gastroenterology: Strain: pag. 8, 15 - Blend: pag. 5, 6, 8, 10, 14 ; Dermatology: Blend: pag. 21 ; Dietetics: Strain: pag. -

The LUCA and Its Complex Virome in Another Recent Synthesis, We Examined the Origins of the Replication and Structural Mart Krupovic , Valerian V
PERSPECTIVES archaea that form several distinct, seemingly unrelated groups16–18. The LUCA and its complex virome In another recent synthesis, we examined the origins of the replication and structural Mart Krupovic , Valerian V. Dolja and Eugene V. Koonin modules of viruses and posited a ‘chimeric’ scenario of virus evolution19. Under this Abstract | The last universal cellular ancestor (LUCA) is the most recent population model, the replication machineries of each of of organisms from which all cellular life on Earth descends. The reconstruction of the four realms derive from the primordial the genome and phenotype of the LUCA is a major challenge in evolutionary pool of genetic elements, whereas the major biology. Given that all life forms are associated with viruses and/or other mobile virion structural proteins were acquired genetic elements, there is no doubt that the LUCA was a host to viruses. Here, by from cellular hosts at different stages of evolution giving rise to bona fide viruses. projecting back in time using the extant distribution of viruses across the two In this Perspective article, we combine primary domains of life, bacteria and archaea, and tracing the evolutionary this recent work with observations on the histories of some key virus genes, we attempt a reconstruction of the LUCA virome. host ranges of viruses in each of the four Even a conservative version of this reconstruction suggests a remarkably complex realms, along with deeper reconstructions virome that already included the main groups of extant viruses of bacteria and of virus evolution, to tentatively infer archaea. We further present evidence of extensive virus evolution antedating the the composition of the virome of the last universal cellular ancestor (LUCA; also LUCA. -
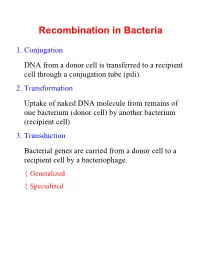
Recombination in Bacteria
Recombination in Bacteria 1. Conjugation DNA from a donor cell is transferred to a recipient cell through a conjugation tube (pili). 2. Transformation Uptake of naked DNA molecule from remains of one bacterium (donor cell) by another bacterium (recipient cell). 3. Transduction Bacterial genes are carried from a donor cell to a recipient cell by a bacteriophage. { Generalized { Specialized Conjugation Ability to conjugate located on the F-plasmid F+ Cells act as donors F- Cells act as recipients F+/F- Conjugation: { F Factor “replicates off” a single strand of DNA. { New strand goes through pili to recipient cell. { New strand is made double stranded. { If entire F-plasmid crosses, then recipient cell becomes F+, otherwise nothing happens Conjugation with Hfr Hfr cell (High Frequency Recombination) cells have F-plasmid integrated into the Chromosome. Integration into the Chromosome is unique for each F-plasmid strain. When F-plasmid material is replicated and sent across pili, Chromosomal material is included. (Figure 6.10 in Klug & Cummings) When chromosomal material is in recipient cell, recombination can occur: { Recombination is double stranded. { Donor genes are recombined into the recipient cell. { Corresponding genes from recipient cell are recombined out of the chromosome and reabsorbed by the cell. Interrupted Mating Mapping 1. Allow conjugation to start Genes closest to the origin of replication site (in the direction of replication) are moved through the pili first. 2. After a set time, interrupt conjugation Only those genes closest to the origin of replication site will conjugate. The long the time, the more that is able to conjugate. 3.