Comprehensive Analysis of Mobile Genetic Elements in the Gut Microbiome Reveals Phylum-Level Niche-Adaptive Gene Pools
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Adaptive Introgression Between Anopheles Sibling Species Eliminates a Major Genomic Island but Not Reproductive Isolation
ARTICLE Received 22 Feb 2014 | Accepted 28 May 2014 | Published 25 Jun 2014 DOI: 10.1038/ncomms5248 OPEN Adaptive introgression between Anopheles sibling species eliminates a major genomic island but not reproductive isolation Chris S. Clarkson1,*, David Weetman1,*, John Essandoh1,2, Alexander E. Yawson2,3, Gareth Maslen4, Magnus Manske4, Stuart G. Field5, Mark Webster6, Tiago Anta˜o1, Bronwyn MacInnis4, Dominic Kwiatkowski4,7 & Martin J. Donnelly1,4 Adaptive introgression can provide novel genetic variation to fuel rapid evolutionary responses, though it may be counterbalanced by potential for detrimental disruption of the recipient genomic background. We examine the extent and impact of recent introgression of a strongly selected insecticide-resistance mutation (Vgsc-1014F) located within one of two exceptionally large genomic islands of divergence separating the Anopheles gambiae species pair. Here we show that transfer of the Vgsc mutation results in homogenization of the entire genomic island region (B1.5% of the genome) between species. Despite this massive dis- ruption, introgression is clearly adaptive with a dramatic rise in frequency of Vgsc-1014F and no discernable impact on subsequent reproductive isolation between species. Our results show (1) how resilience of genomes to massive introgression can permit rapid adaptive response to anthropogenic selection and (2) that even extreme prominence of genomic islands of divergence can be an unreliable indicator of importance in speciation. 1 Department of Vector Biology, Liverpool School of Tropical Medicine, Pembroke Place, Liverpool L3 5QA, UK. 2 Cape Coast Department of Entomology and Wildlife, School of Biological Science, University of Cape Coast, Cape Coast, Ghana. 3 Biotechnology and Nuclear Agriculture Research Institute, Ghana Atomic Energy Commission, PO Box LG 80, Legon, Accra, Ghana. -

Mobile Genetic Elements in Streptococci
Curr. Issues Mol. Biol. (2019) 32: 123-166. DOI: https://dx.doi.org/10.21775/cimb.032.123 Mobile Genetic Elements in Streptococci Miao Lu#, Tao Gong#, Anqi Zhang, Boyu Tang, Jiamin Chen, Zhong Zhang, Yuqing Li*, Xuedong Zhou* State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, PR China. #Miao Lu and Tao Gong contributed equally to this work. *Address correspondence to: [email protected], [email protected] Abstract Streptococci are a group of Gram-positive bacteria belonging to the family Streptococcaceae, which are responsible of multiple diseases. Some of these species can cause invasive infection that may result in life-threatening illness. Moreover, antibiotic-resistant bacteria are considerably increasing, thus imposing a global consideration. One of the main causes of this resistance is the horizontal gene transfer (HGT), associated to gene transfer agents including transposons, integrons, plasmids and bacteriophages. These agents, which are called mobile genetic elements (MGEs), encode proteins able to mediate DNA movements. This review briefly describes MGEs in streptococci, focusing on their structure and properties related to HGT and antibiotic resistance. caister.com/cimb 123 Curr. Issues Mol. Biol. (2019) Vol. 32 Mobile Genetic Elements Lu et al Introduction Streptococci are a group of Gram-positive bacteria widely distributed across human and animals. Unlike the Staphylococcus species, streptococci are catalase negative and are subclassified into the three subspecies alpha, beta and gamma according to the partial, complete or absent hemolysis induced, respectively. The beta hemolytic streptococci species are further classified by the cell wall carbohydrate composition (Lancefield, 1933) and according to human diseases in Lancefield groups A, B, C and G. -

Divergence of a Genomic Island Leads to the Evolution of Melanization in a Halophyte Root Fungus
The ISME Journal https://doi.org/10.1038/s41396-021-01023-8 ARTICLE Divergence of a genomic island leads to the evolution of melanization in a halophyte root fungus 1,2 3 4 5,6 7,8,9 Zhilin Yuan ● Irina S. Druzhinina ● John G. Gibbons ● Zhenhui Zhong ● Yves Van de Peer ● 10 11 2 1,2 12 13 Russell J. Rodriguez ● Zhongjian Liu ● Xinyu Wang ● Huanshen Wei ● Qi Wu ● Jieyu Wang ● 12 3 1,2 14,15 Guohui Shi ● Feng Cai ● Long Peng ● Francis M. Martin Received: 7 October 2020 / Revised: 9 May 2021 / Accepted: 24 May 2021 © The Author(s) 2021. This article is published with open access, corrected publication 2021 Abstract Understanding how organisms adapt to extreme living conditions is central to evolutionary biology. Dark septate endophytes (DSEs) constitute an important component of the root mycobiome and they are often able to alleviate host abiotic stresses. Here, we investigated the molecular mechanisms underlying the beneficial association between the DSE Laburnicola rhizohalophila and its host, the native halophyte Suaeda salsa, using population genomics. Based on genome-wide Fst (pairwise fixation index) and Vst analyses, which compared the variance in allele frequencies of single-nucleotide polymorphisms (SNPs) and copy number 1234567890();,: 1234567890();,: variants (CNVs), respectively, we found a high level of genetic differentiation between two populations. CNV patterns revealed population-specific expansions and contractions. Interestingly, we identified a ~20 kbp genomic island of high divergence with a strong sign of positive selection. This region contains a melanin-biosynthetic polyketide synthase gene cluster linked to six additional genes likely involved in biosynthesis, membrane trafficking, regulation, and localization of melanin. -

Horizontal Gene Transfer
Genetic Variation: The genetic substrate for natural selection Horizontal Gene Transfer Dr. Carol E. Lee, University of Wisconsin Copyright ©2020; Do not upload without permission What about organisms that do not have sexual reproduction? In prokaryotes: Horizontal gene transfer (HGT): Also termed Lateral Gene Transfer - the lateral transmission of genes between individual cells, either directly or indirectly. Could include transformation, transduction, and conjugation. This transfer of genes between organisms occurs in a manner distinct from the vertical transmission of genes from parent to offspring via sexual reproduction. These mechanisms not only generate new gene assortments, they also help move genes throughout populations and from species to species. HGT has been shown to be an important factor in the evolution of many organisms. From some basic background on prokaryotic genome architecture Smaller Population Size • Differences in genome architecture (noncoding, nonfunctional) (regulatory sequence) (transcribed sequence) General Principles • Most conserved feature of Prokaryotes is the operon • Gene Order: Prokaryotic gene order is not conserved (aside from order within the operon), whereas in Eukaryotes gene order tends to be conserved across taxa • Intron-exon genomic organization: The distinctive feature of eukaryotic genomes that sharply separates them from prokaryotic genomes is the presence of spliceosomal introns that interrupt protein-coding genes Small vs. Large Genomes 1. Compact, relatively small genomes of viruses, archaea, bacteria (typically, <10Mb), and many unicellular eukaryotes (typically, <20 Mb). In these genomes, protein-coding and RNA-coding sequences occupy most of the genomic sequence. 2. Expansive, large genomes of multicellular and some unicellular eukaryotes (typically, >100 Mb). In these genomes, the majority of the nucleotide sequence is non-coding. -

Transduction by Bacteriophage Ti * Henry Drexler
Proceedings of the National Academy of Sciences Vol. 66, No. 4, pp. 1083-1088, August 1970 Transduction by Bacteriophage Ti * Henry Drexler DEPARTMENT OF MICROBIOLOGY, BOWMAN GRAY SCHOOL OF MEDICINE, WAKE FOREST UNIVERSITY, WINSTON-SALEM, NORTH CAROLINA Communicated by Edward L. Tatum, May 25, 1970 Abstract. Amber mutants of the virulent coliphage T1 are able to transduce a wide variety of genetic characteristics from permissive to nonpermissive K strains of Escherichia coli. The virulent coliphage T1 is not known to be related to any temperate phage. Certain of the characteristics of T1 seem to be incompatible with its potential existence as a temperate phage; for example, the average latent period for T1 is only 13 min' and about 70% of its DNA is derived from the host.2 In order to demonstrate transduction by T1 it is necessray to provide condi- tions in which potential transductants are able to survive; this is accomplished by using amber mutants of T1 to transduce nonpermissive recipients. Experi- ments which show that T1 is a transducing phage are presented and have been designed chiefly to illustrate the following points: (1) Infection by T1 is able to cause heritable changes in recipients; (2) the genotype of the donor host is im- portant in determining what characteristics can be transferred to recipients; (3) the changes in the recipients are not caused by transformation; and (4) there is a similarity between the transducing activity and the plaque-forming ability of T1 with respect to serology, host range, and density. Materials and Methods. Bacterial strains: The abbreviations and symbols of Demerec et al.3 and Taylor and Trotter4 are used to describe all pertinent genotypes. -

Estimation of Pathogenic Potential of an Environmental Pseudomonas
www.nature.com/scientificreports OPEN Estimation of pathogenic potential of an environmental Pseudomonas aeruginosa isolate using comparative genomics Carola Berger1, Christian Rückert2, Jochen Blom3, Korneel Rabaey4, Jörn Kalinowski2 & Miriam A. Rosenbaum1,5* The isolation and sequencing of new strains of Pseudomonas aeruginosa created an extensive dataset of closed genomes. Many of the publicly available genomes are only used in their original publication while additional in silico information, based on comparison to previously published genomes, is not being explored. In this study, we defned and investigated the genome of the environmental isolate P. aeruginosa KRP1 and compared it to more than 100 publicly available closed P. aeruginosa genomes. By using diferent genomic island prediction programs, we could identify a total of 17 genomic islands and 8 genomic islets, marking the majority of the accessory genome that covers ~ 12% of the total genome. Based on intra-strain comparisons, we are able to predict the pathogenic potential of this environmental isolate. It shares a substantial amount of genomic information with the highly virulent PSE9 and LESB58 strains. For both of these, the increased virulence has been directly linked to their accessory genome before. Hence, the integrated use of previously published data can help to minimize expensive and time consuming wetlab work to determine the pathogenetic potential. Pseudomonas aeruginosa has been isolated from terrestrial and marine soil, fresh and salt water, sewage, plants, animals, and humans 1. For the latter habitats, it is known as an opportunistic pathogen, which usually spreads to already vulnerable patients, causing ~ 10% of all nosocomial infections in most European Union hospitals2. -

The LUCA and Its Complex Virome in Another Recent Synthesis, We Examined the Origins of the Replication and Structural Mart Krupovic , Valerian V
PERSPECTIVES archaea that form several distinct, seemingly unrelated groups16–18. The LUCA and its complex virome In another recent synthesis, we examined the origins of the replication and structural Mart Krupovic , Valerian V. Dolja and Eugene V. Koonin modules of viruses and posited a ‘chimeric’ scenario of virus evolution19. Under this Abstract | The last universal cellular ancestor (LUCA) is the most recent population model, the replication machineries of each of of organisms from which all cellular life on Earth descends. The reconstruction of the four realms derive from the primordial the genome and phenotype of the LUCA is a major challenge in evolutionary pool of genetic elements, whereas the major biology. Given that all life forms are associated with viruses and/or other mobile virion structural proteins were acquired genetic elements, there is no doubt that the LUCA was a host to viruses. Here, by from cellular hosts at different stages of evolution giving rise to bona fide viruses. projecting back in time using the extant distribution of viruses across the two In this Perspective article, we combine primary domains of life, bacteria and archaea, and tracing the evolutionary this recent work with observations on the histories of some key virus genes, we attempt a reconstruction of the LUCA virome. host ranges of viruses in each of the four Even a conservative version of this reconstruction suggests a remarkably complex realms, along with deeper reconstructions virome that already included the main groups of extant viruses of bacteria and of virus evolution, to tentatively infer archaea. We further present evidence of extensive virus evolution antedating the the composition of the virome of the last universal cellular ancestor (LUCA; also LUCA. -
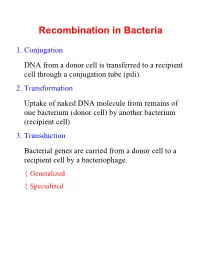
Recombination in Bacteria
Recombination in Bacteria 1. Conjugation DNA from a donor cell is transferred to a recipient cell through a conjugation tube (pili). 2. Transformation Uptake of naked DNA molecule from remains of one bacterium (donor cell) by another bacterium (recipient cell). 3. Transduction Bacterial genes are carried from a donor cell to a recipient cell by a bacteriophage. { Generalized { Specialized Conjugation Ability to conjugate located on the F-plasmid F+ Cells act as donors F- Cells act as recipients F+/F- Conjugation: { F Factor “replicates off” a single strand of DNA. { New strand goes through pili to recipient cell. { New strand is made double stranded. { If entire F-plasmid crosses, then recipient cell becomes F+, otherwise nothing happens Conjugation with Hfr Hfr cell (High Frequency Recombination) cells have F-plasmid integrated into the Chromosome. Integration into the Chromosome is unique for each F-plasmid strain. When F-plasmid material is replicated and sent across pili, Chromosomal material is included. (Figure 6.10 in Klug & Cummings) When chromosomal material is in recipient cell, recombination can occur: { Recombination is double stranded. { Donor genes are recombined into the recipient cell. { Corresponding genes from recipient cell are recombined out of the chromosome and reabsorbed by the cell. Interrupted Mating Mapping 1. Allow conjugation to start Genes closest to the origin of replication site (in the direction of replication) are moved through the pili first. 2. After a set time, interrupt conjugation Only those genes closest to the origin of replication site will conjugate. The long the time, the more that is able to conjugate. 3. -

Microbial Genetics by Dr Preeti Bajpai
Dr. Preeti Bajpai Genes: an overview ▪ A gene is the functional unit of heredity ▪ Each chromosome carry a linear array of multiple genes ▪ Each gene represents segment of DNA responsible for synthesis of RNA or protein product ▪ A gene is considered to be unit of genetic information that controls specific aspect of phenotype DNA Chromosome Gene Protein-1 Prokaryotic Courtesy: Team Shrub https://twitter.com/realscientists/status/927 cell 667237145767937 Genetic exchange within Prokaryotes The genetic exchange occurring in bacteria involve transfers of genes from one bacterium to another. The gene transfer in prokaryotic cells is thus unidirectional and the recombination events usually occur between a fragment of one chromosome (from a donor cell) and a complete chromosome (in a recipient cell) Mechanisms for genetic exchange Bacteria exchange genetic material through three different parasexual processes* namely transformation, conjugation and transduction. *Parasexual process involves recombination of genes from genetically distinct cells occurring without involvement of meiosis and fertilization Principles of Genetics-sixth edition Courtesy: Beatrice the Biologist.com by D. Peter Snustad & Michael J. Simmons (http://www.beatricebiologist.com/2014/08/bacterial-gifts/) Transformation: an introduction Transformation involves the uptake of free DNA molecules released from one bacterium (the donor cell) by another bacterium (the recipient cell). Frederick Griffith discovered transformation in Streptococcus pneumoniae (pneumococcus) in 1928. In his experiments, Griffith used two related strains of bacteria, known as R and S. The R bacteria (nonvirulent) formed colonies, or clumps of related bacteria, that Frederick Griffith 1877-1941 had a rough appearance (hence the abbreviation "R"). The S bacteria (virulent) formed colonies that were rounded and smooth (hence the abbreviation "S"). -

Horizontal Gene Transfer Elements: Plasmids in Antarctic Microorganisms
Chapter 5 Horizontal Gene Transfer Elements: Plasmids in Antarctic Microorganisms Matías Giménez, Gastón Azziz, Paul R. Gill, and Silvia Batista Abstract Plasmids play an important role in the evolution of microbial communi- ties. These mobile genetic elements can improve host survival and may also be involved in horizontal gene transfer (HGT) events between individuals. Diverse culture-dependent and culture-independent approaches have been used to character- ize these mobile elements. Culture-dependent methods are usually associated with classical microbiological techniques. In the second approach, development of spe- cific protocols for analysis of metagenomes involves many challenges, including assembly of sequences and availability of a reliable database, which are crucial. In addition, alternative strategies have been developed for the characterization of plas- mid DNA in a sample, generically referred to as plasmidome. The Antarctic continent has environments with diverse characteristics, including some with very low temperatures, humidity levels, and nutrients. The presence of microorganisms and genetic elements capable of being transferred horizontally has been confirmed in these environments, and it is generally accepted that some of these elements, such as plasmids, actively participate in adaptation mechanisms of host microorganisms. Information related to structure and function of HGT elements in Antarctic bac- teria is very limited compared to what is known about HGT in bacteria from temper- ate/tropical environments. Some studies are done with biotechnological objectives. The search for mobile elements, such as plasmids, may be related to improve the expression of heterologous genes in host organisms growing at very low tempera- tures. More recently, however, additional studies have been done to detect plasmids in isolates, associated or not with specific phenotypes such as drug resistance. -

The Association of Group IIB Intron with Integrons in Hypersaline Environments Sarah Sonbol1 and Rania Siam1,2*
Sonbol and Siam Mobile DNA (2021) 12:8 https://doi.org/10.1186/s13100-021-00234-2 RESEARCH Open Access The association of group IIB intron with integrons in hypersaline environments Sarah Sonbol1 and Rania Siam1,2* Abstract Background: Group II introns are mobile genetic elements used as efficient gene targeting tools. They function as both ribozymes and retroelements. Group IIC introns are the only class reported so far to be associated with integrons. In order to identify group II introns linked with integrons and CALINS (cluster of attC sites lacking a neighboring integron integrase) within halophiles, we mined for integrons in 28 assembled metagenomes from hypersaline environments and publically available 104 halophilic genomes using Integron Finder followed by blast search for group II intron reverse transcriptases (RT)s. Results: We report the presence of different group II introns associated with integrons and integron-related sequences denoted by UHB.F1, UHB.I2, H.ha.F1 and H.ha.F2. The first two were identified within putative integrons in the metagenome of Tanatar-5 hypersaline soda lake, belonging to IIC and IIB intron classes, respectively at which the first was a truncated intron. Other truncated introns H.ha.F1 and H.ha.F2 were also detected in a CALIN within the extreme halophile Halorhodospira halochloris, both belonging to group IIB introns. The intron-encoded proteins (IEP) s identified within group IIB introns belonged to different classes: CL1 class in UHB.I2 and bacterial class E in H.ha.Fa1 and H.ha.F2. A newly identified insertion sequence (ISHahl1)ofIS200/605 superfamily was also identified adjacent to H. -

A New Biological Definition of Life
BioMol Concepts 2020; 11: 1–6 Research Article Open Access Victor V. Tetz, George V. Tetz* A new biological definition of life https://doi.org/10.1515/bmc-2020-0001 received August 17, 2019; accepted November 22, 2019. new avenues for drug development and prediction of the results of genetic interventions. Abstract: Here we have proposed a new biological Defining life is important to understand the definition of life based on the function and reproduction development and maintenance of living organisms of existing genes and creation of new ones, which is and to answer questions on the origin of life. Several applicable to both unicellular and multicellular organisms. definitions of the term “life” have been proposed (1-14). First, we coined a new term “genetic information Although many of them are highly controversial, they are metabolism” comprising functioning, reproduction, and predominantly based on important biological properties creation of genes and their distribution among living and of living organisms such as reproduction, metabolism, non-living carriers of genetic information. Encompassing growth, adaptation, stimulus responsiveness, genetic this concept, life is defined as organized matter that information inheritance, evolution, and Darwinian provides genetic information metabolism. Additionally, approach (1-5, 15). we have articulated the general biological function of As suggested by the Nobel Prize-winning physicist, life as Tetz biological law: “General biological function Erwin Schrödinger, in his influential essay What Is of life is to provide genetic information metabolism” and Life ?, the purpose of life relies on creating an entropy, formulated novel definition of life: “Life is an organized and therefore defined living things as not just a “self- matter that provides genetic information metabolism”.