Technical/Application Article 02 Version 1.15 21St May 2020 WRH/FD/NJG
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
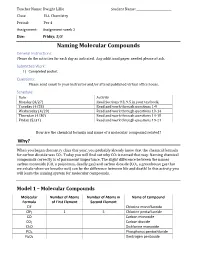
Naming Molecular Compounds General Instructions: Please Do the Activities for Each Day As Indicated
Teacher Name: Dwight Lillie Student Name: ________________________ Class: ELL Chemistry Period: Per 4 Assignment: Assignment week 2 Due: Friday, 5/8 Naming Molecular Compounds General Instructions: Please do the activities for each day as indicated. Any additional paper needed please attach. Submitted Work: 1) Completed packet. Questions: Please send email to your instructor and/or attend published virtual office hours. Schedule: Date Activity Monday (4/27) Read Sections 9.3, 9.5 in your textbook. Tuesday (4/28) Read and work through questions 1-9 Wednesday (4/29) Read and work through questions 10-14 Thursday (4/30) Read and work through questions 14-18 Friday (5/31) Read and work through questions 19-21 How are the chemical formula and name of a molecular compound related? Why? When you began chemistry class this year, you probably already knew that the chemical formula for carbon dioxide was CO2. Today you will find out why CO2 is named that way. Naming chemical compounds correctly is of paramount importance. The slight difference between the names carbon monoxide (CO, a poisonous, deadly gas) and carbon dioxide (CO2, a greenhouse gas that we exhale when we breathe out) can be the difference between life and death! In this activity you will learn the naming system for molecular compounds. Model 1 – Molecular Compounds Molecular Number of Atoms Number of Atoms in Name of Compound Formula of First Element Second Element ClF Chlorine monofluoride ClF5 1 5 Chlorine pentafluoride CO Carbon monoxide CO2 Carbon dioxide Cl2O Dichlorine monoxide PCl5 Phosphorus pentachloride N2O5 Dinitrogen pentoxide 1. Fill in the table to indicate the number of atoms of each type in the molecular formula. -

Health-Based Reassessment of Administrative Occupational Exposure Limits
Gezondheidsraad Voorzitter Health Council of the Netherlands Aan de Staatssecretaris van Sociale Zaken en Werkgelegenheid Onderwerp : Aanbieding adviezen herevaluatie bestuurlijke MAC-waarden Uw kenmerk : ARBO/AMIL/97/00648 Ons kenmerk : U 2204/CB/mj/563-B5 Bijlagen : 1 Datum : 13 november 2001 Mijnheer de staatssecretaris, Op verzoek van uw ambtsvoorganger bied ik u hierbij 12 adviezen aan van een reeks over de gezondheidskundige basis van uit het buitenland overgenomen grenswaarden voor beroepsmatige blootstelling aan stoffen. Het verzoek om deze adviezen is in algemene zin vervat in brief nr ARBO/AMIL/97/00648 en in latere stadia door uw departement toegespitst op afzonderlijke stoffen. De adviezen zijn opgesteld door een daartoe door mij geformeerde internationale commissie van de Gezondheidsraad en beoordeeld door de Beraadsgroep Gezondheid en Omgeving. De beoogde reeks van in het Engels gestelde adviezen zal losbladig worden gepubliceerd onder ons publicatienummer 2000/15OSH en, conform de aan de Gezondheidsraad voorgelegde toespitsingen van de adviesaanvraag, betrekking hebben op 168 stoffen. Het u thans aangeboden tweede pakket bestaat uit de adviezen genummerd 2000/15OSH/018 tot en met 2000/15OSH/029, respectievelijk betrekking hebbend op: bornanon-2 (kamfer, synthetisch), chloortrifluoride, o-chloorstyreen, cyclohexylamine, dizwaveldecafluoride, hexafluoroaceton, keteen, pentachloornaftaleen, perchlorylfluoride, m-ftalodinitril, thionylchloride en trichloornaftaleen. Bij afronding van de werkzaamheden van de hierboven bedoelde -
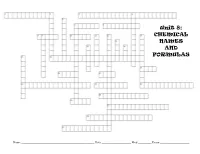
Unit 8: CHEMICAL NAMES and FORMULAS
Unit 8: CHEMICAL NAMES AND FORMULAS Name _________________________________________________________________ Date __________________________ Mod ____________ Exam __________________________ Across 28. These have various oxidation numbers 1. These have a 1+ oxidation number 29. The name for the compound of calcium and oxygen 3. Every compound always contains the same elements in the same proportions. Down 6. The type of compound containing only two elements 2. The number that tells how many atoms of an element are in a 7. Compounds form so that atoms can satisfy what rule? unit of the compound 8. ammonium chloride 4. A compound containing more than 2 different elements 10. The formula for chromium (III) and oxygen 5. A covalent bond wherein the electrons are shared equally 18. Name P4O10 among elements 19. silver oxide 6. The name for a compound of barium and hydroxide 20. A covalent bond wherein the electrons are not shared equally 9. These have a 1- oxidation number 21. The formula for beryllium and iodine 11. Nickel (I) carbonate 22. NH4OH 12. Name As2O5 23. A chemical combination of two or more elements having 13. The group which does not have oxidation numbers. different properties than the individual elements 14. Nona is the prefix for ___ in a covalent bond 24. Name Na2S 15. The prefix for 7 in a covalent bond 25. A single atom with a charge 16. The formula for calcium and oxygen 26. The prefix for 4 in a covalent bond 17. The ion ClO3- 27. The positive/negative number assigned to an element that shows its ability to combine in a compound Also for the exam: Be able to draw electron dot diagrams for various ionic bonds (brackets) and covalent bonds (dots). -

1. in an Ionic Compound of Formula Mmxn, the Metal (M) Occupies a Face-Centered Cubic Lattice and the Anions Occupy Four Positions Inside the Unit Cell
Name: __________________________ Date: _____________ 1. In an ionic compound of formula MmXn, the metal (M) occupies a face-centered cubic lattice and the anions occupy four positions inside the unit cell. Determine the formula of the compound. A) MX B) MX2 C) M2X D) MX3 E) M3X 2. Which one of the following is not a property of gases? A) particles in definite positions B) relatively low densities C) fills any container completely D) expand upon heating E) easily compressed 3. Give the systematic name for the following binary compound: S2F10 A) Disulfur decafluoride B) Sulfur fluoride C) Sulfur decafluoride D) Disulfur pentafluoride E) Disulfur fluoride 4. Which one of the following is not one of the hypotheses of Dalton's atomic theory? A) Atoms are composed of protons, neutrons, and electrons. B) All atoms of a given element are identical; the atoms of different elements are different and have different properties. C) Atoms of an element are not changed into different types of atoms by chemical reactions: atoms are neither created nor destroyed in chemical reactions. D) Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms. E) Each element is composed of extremely small particles called atoms. Page 1 5. What is the molar mass (g/mol) of sodium sulfite? A) 126.05 B) 142.05 C) 119.05 D) 103.05 E) 149.03 6. Ammonia reacts with oxygen gas to form nitric oxide (NO) and water vapour as follows: 4NH3 + 5O2 → 4NO + 6H2O What is the maximum amount of water that may be produced if 40.0 g NH3 and 50.0 g O2 are mixed and allowed to react? A) 1.88 mol B) 1.56 mol C) 3.52 mol D) 3.91 mol E) 2.35 mol 7. -

Things to Be Done
DRAFT MAY 2003 ANNEX 1: CHEMICAL AGENTS 1. Introduction The large-scale use of toxic chemicals as weapons first became possible during the First World War (1914–1918) thanks to the growth of the chemical industry. More than 110 000 tonnes were disseminated over the battlefields, the greater part on the western front. Initially, the chemicals were used, not to cause casualties in the sense of putting enemy combatants out of action, but rather to harass. Though the sensory irritants used were powerful enough to disable those who were exposed to them, they served mainly to drive enemy combatants out of the trenches or other cover that protected them from conventional fire, or to disrupt enemy artillery or supplies. About 10% of the total tonnage of chemical warfare agents used during the war were chemicals of this type, namely lacrimators (tear gases), sternutators and vomiting agents. However, use of more lethal chemicals soon followed the introduction of disabling chemicals. In all, chemical agents caused some 1.3 million casualties, including 90 000 deaths. During the First World War, almost every known noxious chemical was screened for its potential as a weapon, and this process was repeated during the Second World War (1939–1945), when substantial stocks of chemical weapons were accumulated, although rarely used in military operations. Between the two world wars, a high proportion of all the new compounds that had been synthesized, or isolated from natural materials, were examined to determine their utility as lethal or disabling chemical weapons. After 1945, these systematic surveys continued, together with a search for novel agents based on advances in biochemistry, toxicology and pharmacology. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

(12) United States Patent (10) Patent No.: US 9,354.231 B1 Glover (45) Date of Patent: May 31, 2016
USOO9354231B1 (12) United States Patent (10) Patent No.: US 9,354.231 B1 Glover (45) Date of Patent: May 31, 2016 (54) REACTIVE SELF-INDICATING ABSORBENT Chemisorption of Cyanogen Chloride by Spinel Ferrite Magnetic Nanoparticles T. Grant Glover, Jared B. DeCoste, Daniel Sabo, and Z. MATERIALS, METHODS, AND SYSTEMS John Zhang Langmuir 2013, 29, 5500-5507.* Glover, T. Grant, et al., “Carbon-Silica Composite Adsorbent: Char (75) Inventor: Thomas Grant Glover, Bel Air, MD acterization and Adsorption of Light Gases.” Microporous and (US) Mesoporous Materials, 111, pp. 1-11, 2008. Peterson, Gregory W., et al., “Ammonia Vapor Removal by (73) Assignee: Leidos, Inc., Reston, VA (US) Cu(BTC) and Its Characterization by MAS NMR.” Journal of Physical Chemistry C, 113, pp. 13906-13917, 2009. Britt, David, et al., “Highly Efficient Separation of Carbon Dioxide (*) Notice: Subject to any disclaimer, the term of this by a Metal-Organic Framework Replete With Open Metal Sites.” patent is extended or adjusted under 35 Proceedings of the National Academy of Science, vol. 106, No. 49. U.S.C. 154(b) by 1165 days. pp. 20637-20640, Dec. 8, 2009. Britt, David, et al., “Metal-Organic Frameworks With High Capacity (21) Appl. No.: 13/189,736 and Selectivity for Harmful Gases.” Proceedings of the National Academy of Science, vol. 105, No. 33, pp. 11623-11627. Aug. 19, (22) Filed: Jul. 25, 2011 2008. * cited by examiner (51) Int. Cl. GOIN 27/2403 (2006.01) Primary Examiner — Krishnan S Menon GOIN33/543 (2006.01) Assistant Examiner — Dwan A Gerido (52) U.S. Cl. (74) Attorney, Agent, or Firm —Dawn-Marie Bey; Bey & CPC ......... -

ORNL Review, Vol
ON THE COVER John Bates examines a thin-film lithium battery in the light of the nitrogen glow discharge in the vacuum deposition chamber used to produce microbatteries at ORNL's Solid State Division. The nitrogen gas reacts with lithium orthophosphate to form a new battery electrolyte film discovered at ORNL. See the article on p. 46. Photo by Bill Norris. The Oak Ridge National Laboratory Review is published quarterly and distributed to employees and others associated with ORNL. The address of the editorial office is Building 4500-South, M.S. 6144, Oak Ridge, TN 37831-6144. Telephone: internal, 4-7183 or 4-6974; commercial, (615) 574-7183 or (615) 574-6974; FfS, 624-7183 or 624-6974. If you have changed your address and want to remain on the mailing list, please notify the editorial office. ORNL is managed by Martin Marietta Energy Systems, Inc. for the Department of Energy under contract DE-AC05-840R21400 Printed in the United States of America. Available from National Technical Information Service, U.S. Department of Commerce, 5285 Port Royal Road, Springfield, VA 22161 . ISSN 0048-1262 Editor Carolyn Krause Associate Editor Jim Pearce Consulting Editor Bill Appleton Designer Vickie Conner Technical Editing Mike Aaron Electronic Publishing Bob Eldridge Photography, Graphic Arts, and Printing and Duplicating departments Volume 25 Number Two, 1992 2 State of the Laboratory-1991: Strengthening R&D Alvin Trivelpiece Organizational changes and enhanced computing capabilities should bolster ORNL's R&D. 28 An Eye on Reactor and Computer Control Jack Schryver and Bill Knee Eye-gaze measurement technology improved by ORNL software may have fascinating uses. -

WO 2015/200485 Al 30 December 2015 (30.12.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/200485 Al 30 December 2015 (30.12.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every G01N 1/22 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/US20 15/037423 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 24 June 2015 (24.06.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 62/016,739 25 June 2014 (25.06.2014) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant: BATTELLE MEMORIAL INSTITUTE GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, [US/US]; 505 King Avenue, Columbus, Ohio 43201-2696 TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (US). -

Investigation of S2fl0 Production and Mitigation in Colllpressed SF6-Insulated Power Systellls
Investigation of S2Fl0 Production and Mitigation in COlllpressed SF6-Insulated Power Systellls D. R. James (Editor), 1. Sauers, and G. D. Griffin, Oak Ridge National Laboratory, R. J. Van Brunt, J. K. Olthoff, and K. L. Stricklett, National Institute of Standards and Technology, F. Y. Chu, J. R. Robins, and H. D. Morrison, Ontario Hydro ResearchDivision Introduction Information on SF6 by-products such as S2F10 can be used to assess potential cooperative Research and Development Agree- ment (CRADA) has been established to study hazards and define safe gas handling and A the production and mitigation of S2FlO(disulfur operatingprocedures. decafluoride), one of a number of toxic by-products formed in electrical discharges in the insulating gas SF6. The particular concern for S2FlOis due to its highly toxic Voltage Electric Systems (CIGRE) [5] has reviewed the nature, the Ceiling Limit Value being 10parts per billion literature on SF6decomposition and has recommended (ppb, or 1 part in 108)and the need for development of that the International Electrotechnical Commission sensitive detection techniques down to this level. There are a large number of SF6 gas-insulated electric power (1EC)modify or make new standards or guidelines for utility personnel to handle and dispose of the arc by- systems already in service in North America, particu- larly circuit breakers. SF6 is extensively used as an products using the CIGRE document as a basis. Although much is known about SF6by-products and insulation and current interruption medium in power circuit breakers, compressed gas transmission lines and their formation in typical power system environments, various components in substations. -

SF6 By-Products: Safety, Cleaning, and Disposal Concerns
SF6 By-products: Safety, Cleaning, and Disposal Concerns U.S. EPA’s International Conference on SF6 and the Environment November 29, 2006 San Antonio, TX Mollie Averyt, ICF International [email protected] 202-862-1569 SF6 Gas Properties • Slow reacting with a relatively high molecular weight and extremely stable molecular structure. • Excellent insulation properties, strong arc quenching abilities, and high dielectric strength • Non-flammable and non-toxic to humans • Colorless and Odorless • Under high temperature conditions (> 350° F), SF6 decomposes into products that are toxic and corrosive 2 SF6 Decomposition and Contamination • Reactive decomposition byproducts form when SF6 is exposed to: 1. spark discharges, 2. partial discharges, 3. switching arcs, and 4. failure arcing • Decomposition byproducts can take the form of gas or powders • Other types of contaminants can include moisture and air (from handling or leakage), dust and particles (mechanical generation) 3 SF6 Decomposition Byproducts Chemical Name Chemical Formula Gaseous Byproducts Sulfur Dioxide SO2 Thionyl Sulfide (sulfur tetrafluoride) SOF2 (SF4) Hydrogen Fluoride HF Disulfur Decafluoride (sulfur pentafluoride) S2F10 (SF5) Sulfuryl Fluoride SO2F2 a Sulfur Tetrafluoride Oxide SOF4 (SF4) a SF4 is readily hydrolyzed to SOF2. Powder Byproducts Tungsten, aluminum, copper fluorides WF6, WO3, AlF3, CuF4 2 Human Health Concerns • Irritating to the eyes, nose, and throat, pulmonary edema and other lung damage, skin and eye burns, nasal congestion, bronchitis; powders may cause -

Disulfur Decafluoride
Disulfur decafluoride (CAS reg no: 5714-22-7) Health-based Reassessment of Administrative Occupational Exposure Limits Committee on Updating of Occupational Exposure Limits, a committee of the Health Council of the Netherlands No. 2000/15OSH/022, The Hague, 13 November 2001 022-1 Preferred citation: Health Council of the Netherlands: Committee on Updating of Occupational Exposure Limits. Disulfur decafluoride; Health-based Reassessment of Administrative Occupational Exposure Limits. The Hague: Health Council of the Netherlands, 2001; 2000/15OSH/022. all rights reserved 022-2 1 Introduction The present document contains the assessment of the health hazard of disulfur decafluoride by the Committee on Updating of Occupational Exposure Limits, a committee of the Health Council of the Netherlands. The first draft of this document was prepared by MA Maclaine Pont, M.Sc. (Wageningen University, Wageningen, the Netherlands). The evaluation of the toxicity of disulfur decafluoride has been based on the review by the American Conference of Governmental Industrial Hygienists (ACG99). Where relevant, the original publications were reviewed and evaluated as will be indicated in the text. In addition, literature was retrieved from the data bases Medline, Toxline, and Chemical Abstracts covering the period of 1966 until November 1999, 1981 until July 1999, and 1937 until September 1999, respectively, and using the following key words: sulfur pentafluoride, sulfur decafluoride, disulfurdecafluoride, sulphur pentafluoride, sulphur decafluoride, disulphurdecafluoride, sulfur fluoride (S2F10), and/or 5714-22-7. The final literature search has been carried out in November 1999. In April 2001, the President of the Health Council released a draft of the document for public review. The committee received no comments.