ORNL Review, Vol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Primary Energy Use and Environmental Effects of Electric Vehicles
Article Primary Energy Use and Environmental Effects of Electric Vehicles Efstathios E. Michaelides Department of Engineering, TCU, Fort Worth, TX 76132, USA; [email protected] Abstract: The global market of electric vehicles has become one of the prime growth industries of the 21st century fueled by marketing efforts, which frequently assert that electric vehicles are “very efficient” and “produce no pollution.” This article uses thermodynamic analysis to determine the primary energy needs for the propulsion of electric vehicles and applies the energy/exergy trade-offs between hydrocarbons and electricity propulsion of road vehicles. The well-to-wheels efficiency of electric vehicles is comparable to that of vehicles with internal combustion engines. Heat transfer to or from the cabin of the vehicle is calculated to determine the additional energy for heating and air-conditioning needs, which must be supplied by the battery, and the reduction of the range of the vehicle. The article also determines the advantages of using fleets of electric vehicles to offset the problems of the “duck curve” that are caused by the higher utilization of wind and solar energy sources. The effects of the substitution of internal combustion road vehicles with electric vehicles on carbon dioxide emission avoidance are also examined for several national electricity grids. It is determined that grids, which use a high fraction of coal as their primary energy source, will actually increase the carbon dioxide emissions; while grids that use a high fraction of renewables and nuclear energy will significantly decrease their carbon dioxide emissions. Globally, the carbon dioxide emissions will decrease by approximately 16% with the introduction of electric vehicles. -

Electric Battery Car Competition Rules
Colorado Middle School Car Competition Electric Battery Division The Colorado Middle School Car Competition is a classroom-based, hands-on educational program for 6th – 8th grade students. Student teams apply math, science, and creativity to construct and race model lithium-ion powered cars. The primary goals of the programs are to: • Generate enthusiasm for science and engineering at a crucial stage in the educational development of young people; • Improve students' understanding of scientific concepts and renewable energy technologies; and • Encourage young people to consider technical careers at an early age. Program description: • Students use mathematics and science principles together with their creativity in a fun, hands-on educational program. • Using engineering principles, students get excited about generating ideas in a group and then building and modifying models based on these ideas. • Students can see for themselves how changes in design are reflected in car performance. • Students work together on teams to apply problem solving and project management skills. The car competition challenges students to use scientific know-how, creative thinking, experimentation, and teamwork to design and build high-performance model electric battery vehicles. Rules Competition Structure: The Colorado competition will use preliminary time trials before progressing to a double elimination tournament for the finals. Each team will have three time trials to achieve their fastest time. Any car that does not finish in 40 seconds will be considered a Did Not Finish (DNF). Only the fastest 16 teams will progress to the double elimination tournament. In the event of a tie, teams will have a race-off to qualify for the double elimination round. -

Modeling, Testing and Economic Analysis of Wind-Electric Battery
NREL/CP-500-24920 · UC Category: 1213 Modeling, Testing and Economic Analysis of a Wind-Electric Battery Charging Station Vahan Gevorgian, David A. Corbus, Stephen Drouilhet, Richard Holz National Renewable Energy Laboratory Karen E. Thomas University of California at Berkeley Presented at Windpower '98 Bakersfield, CA April 27-May 1, 1998 National Renewable Energy Laboratory 1617 Cole Boulevard Golden, Colorado 80401-3393 A national laboratory of the U.S. Department of Energy Managed by Midwest Research Institute for the U.S. Department of Energy under contract No. DE-AC36-83CH10093 Work performed under task number WE802230 July 1998 NOTICE This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercialproduct, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or any agency thereof. The views and opinions of authord expressed herein do not necessarily state or reflect those of the United States government or any agency thereof. Available to DOE and DOE contractors from: Office of Scientific and Technical Information (OSTI) P.O. Box 62 Oak Ridge, TN 37831 Prices available by calling (423) 576-8401 Available to the public from: National Technical Information Service (NTIS) U.S. -

DESIGN of a WATER TOWER ENERGY STORAGE SYSTEM a Thesis Presented to the Faculty of Graduate School University of Missouri
DESIGN OF A WATER TOWER ENERGY STORAGE SYSTEM A Thesis Presented to The Faculty of Graduate School University of Missouri - Columbia In Partial Fulfillment of the Requirements for the Degree Master of Science by SAGAR KISHOR GIRI Dr. Noah Manring, Thesis Supervisor MAY 2013 The undersigned, appointed by the Dean of the Graduate School, have examined he thesis entitled DESIGN OF A WATER TOWER ENERGY STORAGE SYSTEM presented by SAGAR KISHOR GIRI a candidate for the degree of MASTER OF SCIENCE and hereby certify that in their opinion it is worthy of acceptance. Dr. Noah Manring Dr. Roger Fales Dr. Robert O`Connell ACKNOWLEDGEMENT I would like to express my appreciation to my thesis advisor, Dr. Noah Manring, for his constant guidance, advice and motivation to overcome any and all obstacles faced while conducting this research and support throughout my degree program without which I could not have completed my master’s degree. Furthermore, I extend my appreciation to Dr. Roger Fales and Dr. Robert O`Connell for serving on my thesis committee. I also would like to express my gratitude to all the students, professors and staff of Mechanical and Aerospace Engineering department for all the support and helping me to complete my master’s degree successfully and creating an exceptional environment in which to work and study. Finally, last, but of course not the least, I would like to thank my parents, my sister and my friends for their continuous support and encouragement to complete my program, research and thesis. ii TABLE OF CONTENTS ACKNOWLEDGEMENTS ............................................................................................ ii ABSTRACT .................................................................................................................... v LIST OF FIGURES ....................................................................................................... -

Naming Molecular Compounds General Instructions: Please Do the Activities for Each Day As Indicated
Teacher Name: Dwight Lillie Student Name: ________________________ Class: ELL Chemistry Period: Per 4 Assignment: Assignment week 2 Due: Friday, 5/8 Naming Molecular Compounds General Instructions: Please do the activities for each day as indicated. Any additional paper needed please attach. Submitted Work: 1) Completed packet. Questions: Please send email to your instructor and/or attend published virtual office hours. Schedule: Date Activity Monday (4/27) Read Sections 9.3, 9.5 in your textbook. Tuesday (4/28) Read and work through questions 1-9 Wednesday (4/29) Read and work through questions 10-14 Thursday (4/30) Read and work through questions 14-18 Friday (5/31) Read and work through questions 19-21 How are the chemical formula and name of a molecular compound related? Why? When you began chemistry class this year, you probably already knew that the chemical formula for carbon dioxide was CO2. Today you will find out why CO2 is named that way. Naming chemical compounds correctly is of paramount importance. The slight difference between the names carbon monoxide (CO, a poisonous, deadly gas) and carbon dioxide (CO2, a greenhouse gas that we exhale when we breathe out) can be the difference between life and death! In this activity you will learn the naming system for molecular compounds. Model 1 – Molecular Compounds Molecular Number of Atoms Number of Atoms in Name of Compound Formula of First Element Second Element ClF Chlorine monofluoride ClF5 1 5 Chlorine pentafluoride CO Carbon monoxide CO2 Carbon dioxide Cl2O Dichlorine monoxide PCl5 Phosphorus pentachloride N2O5 Dinitrogen pentoxide 1. Fill in the table to indicate the number of atoms of each type in the molecular formula. -

The Pennsylvania State University Schreyer Honors College
THE PENNSYLVANIA STATE UNIVERSITY SCHREYER HONORS COLLEGE SCHOOL OF SCIENCE, ENGINEERING AND TECHNOLOGY EFFECT ON CHARGING EFFICIENCY USING GALLIUM NITRIDE DEVICES THIEN NHIEN HUYNH Fall 2014 A thesis submitted in partial fulfillment of the requirements for a baccalaureate degree in Electrical Engineering with honors in Electrical Engineering Reviewed and approved* by the following: Seth Wolpert, Ph.D Associate Professor of Electrical Engineering Thesis Supervisor Peter Idowu, Ph.D Professor of Electrical Engineering Faculty Reader Ronald Walker, Ph. D Associate Professor of Mathematics Honors Advisor * Signatures are on file in the Schreyer Honors College. i ABSTRACT Electric vehicles (EVs) and hybrid electric vehicles (HEVs) were created to lessen our dependence on natural resources. EVs and HEVs run on battery packs and the pack can be recharged from a household outlet. Because the vehicles are charged using energy draw from the grid, the problem of efficiency on a large scale become a concern. For conventional chargers, the charging efficiency may be improved due to enhanced in battery technology, charging protocol, or charging circuitry. Recently, Gallium Nitride (GaN) devices were introduced that have better performance than other semiconductors used in charging circuits. GaN has a higher bandgap than conventional materials which allows it to withstand high level of voltage. GaN can also operate at higher frequency resulting in much faster switching capability. The ability to withstand higher temperature allows GaN devices to require smaller heat sinks which effectively reduce the cost of the devices. In this thesis, a DC-DC converter as used in battery charger will be designed using Gallium Nitride devices and tested for efficiency. -

Electric Potential and Potential Energy
Electric Potential and Potential Energy Electric Potential Work-energy theorem: Change in potential energy = work done m Higher PE Gravitational Potential Energy It requires a certain amount of work to raise an object • Chapter 17 (Giancoli) of mass m from the ground to some distance above the • All sections except 17.6 (electric m Lower PE ground. dipoles) i.e. We have increased the potential energy of the object. PE=W=Force x Displacement Electric Potential Energy ⎛ kqQ ⎞ Find the work done in bringing a charge q from infinitely ∆ W = − F∆ r = −⎜ 2 ⎟⋅∆ r far away to a distance R from charge Q. ⎝ r ⎠ Q R R ∆r kqQ PE = ∆W = - ∆r F ∫ ∫ r 2 + +q ∞ ∞ R R ⎡1⎤ Charge is moved towards R = kqQ⎢ ⎥ by increments of ∆r ⎣ r ⎦∞ kqQ For a small displacement ∆r, the work done is: PE = *Note: PE = 0 when R ∞ R ∆W = force x displacement = -F • ∆r (we have a negative sign as the direction of the force is This is the PE of a charge q when it is a distance R from Q. opposite to the direction of the displacement) • PE is a scalar quantity If the PE is negative (when the charges have opposite signs), then the work is done by the charge, decreasing its PE. • The sign of the charges must be kept in all calculations Displacement + F q • Depending on the signs of Q and q, the PE could be positive or negative If the PE is positive (when the charges are both positive or both negative), then work must be done on the charge q to bring it closer to Q, increasing its PE. -

Electric Potential Difference Between Two Points
We are used to voltage in our lives—a 12-volt car battery, 110 V or 220 V at home, 1.5-volt flashlight batteries, and so on. Here we see displayed the voltage produced across a human heart, known as an electrocardiogram. Voltage is the same as electric potential difference between two points. Electric potential is defined as the potential energy per unit charge. We discuss voltage and its relation to electric field, as well as electric energy storage, capacitors, and applications including the ECG shown here, binary numbers and digital electronics, TV and computer monitors, and digital TV. P T A E H R C Electric Potential 17 CHAPTER-OPENING QUESTION—Guess now! CONTENTS When two positively charged small spheres are pushed toward each other, what 17–1 Electric Potential Energy and happens to their potential energy? Potential Difference (a) It remains unchanged. 17–2 Relation between Electric (b) It decreases. Potential and Electric Field (c) It increases. 17–3 Equipotential Lines and (d) There is no potential energy in this situation. Surfaces 17–4 The Electron Volt, a Unit of Energy e saw in Chapter 6 that the concept of energy was extremely valuable 17–5 Electric Potential Due to in dealing with the subject of mechanics. For one thing, energy is a Point Charges W conserved quantity and is thus an important tool for understanding *17–6 Potential Due to Electric nature. Furthermore, we saw that many Problems could be solved using the Dipole; Dipole Moment energy concept even though a detailed knowledge of the forces involved was not 17–7 Capacitance possible, or when a calculation involving Newton’s laws would have been too 17–8 Dielectrics difficult. -

Health-Based Reassessment of Administrative Occupational Exposure Limits
Gezondheidsraad Voorzitter Health Council of the Netherlands Aan de Staatssecretaris van Sociale Zaken en Werkgelegenheid Onderwerp : Aanbieding adviezen herevaluatie bestuurlijke MAC-waarden Uw kenmerk : ARBO/AMIL/97/00648 Ons kenmerk : U 2204/CB/mj/563-B5 Bijlagen : 1 Datum : 13 november 2001 Mijnheer de staatssecretaris, Op verzoek van uw ambtsvoorganger bied ik u hierbij 12 adviezen aan van een reeks over de gezondheidskundige basis van uit het buitenland overgenomen grenswaarden voor beroepsmatige blootstelling aan stoffen. Het verzoek om deze adviezen is in algemene zin vervat in brief nr ARBO/AMIL/97/00648 en in latere stadia door uw departement toegespitst op afzonderlijke stoffen. De adviezen zijn opgesteld door een daartoe door mij geformeerde internationale commissie van de Gezondheidsraad en beoordeeld door de Beraadsgroep Gezondheid en Omgeving. De beoogde reeks van in het Engels gestelde adviezen zal losbladig worden gepubliceerd onder ons publicatienummer 2000/15OSH en, conform de aan de Gezondheidsraad voorgelegde toespitsingen van de adviesaanvraag, betrekking hebben op 168 stoffen. Het u thans aangeboden tweede pakket bestaat uit de adviezen genummerd 2000/15OSH/018 tot en met 2000/15OSH/029, respectievelijk betrekking hebbend op: bornanon-2 (kamfer, synthetisch), chloortrifluoride, o-chloorstyreen, cyclohexylamine, dizwaveldecafluoride, hexafluoroaceton, keteen, pentachloornaftaleen, perchlorylfluoride, m-ftalodinitril, thionylchloride en trichloornaftaleen. Bij afronding van de werkzaamheden van de hierboven bedoelde -
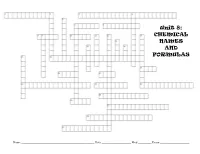
Unit 8: CHEMICAL NAMES and FORMULAS
Unit 8: CHEMICAL NAMES AND FORMULAS Name _________________________________________________________________ Date __________________________ Mod ____________ Exam __________________________ Across 28. These have various oxidation numbers 1. These have a 1+ oxidation number 29. The name for the compound of calcium and oxygen 3. Every compound always contains the same elements in the same proportions. Down 6. The type of compound containing only two elements 2. The number that tells how many atoms of an element are in a 7. Compounds form so that atoms can satisfy what rule? unit of the compound 8. ammonium chloride 4. A compound containing more than 2 different elements 10. The formula for chromium (III) and oxygen 5. A covalent bond wherein the electrons are shared equally 18. Name P4O10 among elements 19. silver oxide 6. The name for a compound of barium and hydroxide 20. A covalent bond wherein the electrons are not shared equally 9. These have a 1- oxidation number 21. The formula for beryllium and iodine 11. Nickel (I) carbonate 22. NH4OH 12. Name As2O5 23. A chemical combination of two or more elements having 13. The group which does not have oxidation numbers. different properties than the individual elements 14. Nona is the prefix for ___ in a covalent bond 24. Name Na2S 15. The prefix for 7 in a covalent bond 25. A single atom with a charge 16. The formula for calcium and oxygen 26. The prefix for 4 in a covalent bond 17. The ion ClO3- 27. The positive/negative number assigned to an element that shows its ability to combine in a compound Also for the exam: Be able to draw electron dot diagrams for various ionic bonds (brackets) and covalent bonds (dots). -

1. in an Ionic Compound of Formula Mmxn, the Metal (M) Occupies a Face-Centered Cubic Lattice and the Anions Occupy Four Positions Inside the Unit Cell
Name: __________________________ Date: _____________ 1. In an ionic compound of formula MmXn, the metal (M) occupies a face-centered cubic lattice and the anions occupy four positions inside the unit cell. Determine the formula of the compound. A) MX B) MX2 C) M2X D) MX3 E) M3X 2. Which one of the following is not a property of gases? A) particles in definite positions B) relatively low densities C) fills any container completely D) expand upon heating E) easily compressed 3. Give the systematic name for the following binary compound: S2F10 A) Disulfur decafluoride B) Sulfur fluoride C) Sulfur decafluoride D) Disulfur pentafluoride E) Disulfur fluoride 4. Which one of the following is not one of the hypotheses of Dalton's atomic theory? A) Atoms are composed of protons, neutrons, and electrons. B) All atoms of a given element are identical; the atoms of different elements are different and have different properties. C) Atoms of an element are not changed into different types of atoms by chemical reactions: atoms are neither created nor destroyed in chemical reactions. D) Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms. E) Each element is composed of extremely small particles called atoms. Page 1 5. What is the molar mass (g/mol) of sodium sulfite? A) 126.05 B) 142.05 C) 119.05 D) 103.05 E) 149.03 6. Ammonia reacts with oxygen gas to form nitric oxide (NO) and water vapour as follows: 4NH3 + 5O2 → 4NO + 6H2O What is the maximum amount of water that may be produced if 40.0 g NH3 and 50.0 g O2 are mixed and allowed to react? A) 1.88 mol B) 1.56 mol C) 3.52 mol D) 3.91 mol E) 2.35 mol 7. -

An In-Situ Accelerator-Based Diagnostic for Plasma-Material Interactions Science in Magnetic Fusion Devices by Zachary Seth Hartwig B.A
An in-situ accelerator-based diagnostic for plasma-material interactions science in magnetic fusion devices by Zachary Seth Hartwig B.A. in Physics, Boston University (2005) Submitted to the Department of Nuclear Science and Engineering in partial fulfillment of the requirements for the degree of Doctor of Philosophy at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY February 2014 c Massachusetts Institute of Technology 2014. All rights reserved. Author............................................. ................. Department of Nuclear Science and Engineering 21 November 2013 Certified by......................................... ................. Dennis G. Whyte Professor Thesis Supervisor Certified by......................................... ................. Richard C. Lanza Senior Research Scientist Thesis Reader Accepted by......................................... ................ Mujid S. Kazimi TEPCO Professor of Nuclear Engineering Chair, Department Committee on Graduate Students 2 An in-situ accelerator-based diagnostic for plasma-material interactions science in magnetic fusion devices by Zachary Seth Hartwig Submitted to the Department of Nuclear Science and Engineering on 21 November 2013, in partial fulfillment of the requirements for the degree of Doctor of Philosophy Abstract Plasma-material interactions (PMI) in magnetic fusion devices such as fuel retention, ma- terial erosion and redeposition, and material mixing present significant scientific and engi- neering challenges, particularly for the next generation of devices that will move towards reactor-relevant conditions. Achieving an integrated understanding of PMI, however, is severely hindered by a dearth of in-situ diagnosis of the plasma-facing component (PFC) surfaces. To address this critical need, this thesis presents an accelerator-based diagnostic that nondestructively measures the evolution of PFC surfaces in-situ. The diagnostic aims to remotely generate isotopic concentration maps that cover a large fraction of the PFC surfaces on a plasma shot-to-shot timescale.