Worksheet #6 Covalent
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
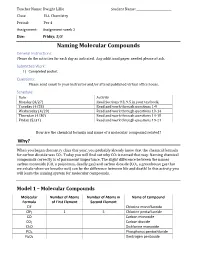
Naming Molecular Compounds General Instructions: Please Do the Activities for Each Day As Indicated
Teacher Name: Dwight Lillie Student Name: ________________________ Class: ELL Chemistry Period: Per 4 Assignment: Assignment week 2 Due: Friday, 5/8 Naming Molecular Compounds General Instructions: Please do the activities for each day as indicated. Any additional paper needed please attach. Submitted Work: 1) Completed packet. Questions: Please send email to your instructor and/or attend published virtual office hours. Schedule: Date Activity Monday (4/27) Read Sections 9.3, 9.5 in your textbook. Tuesday (4/28) Read and work through questions 1-9 Wednesday (4/29) Read and work through questions 10-14 Thursday (4/30) Read and work through questions 14-18 Friday (5/31) Read and work through questions 19-21 How are the chemical formula and name of a molecular compound related? Why? When you began chemistry class this year, you probably already knew that the chemical formula for carbon dioxide was CO2. Today you will find out why CO2 is named that way. Naming chemical compounds correctly is of paramount importance. The slight difference between the names carbon monoxide (CO, a poisonous, deadly gas) and carbon dioxide (CO2, a greenhouse gas that we exhale when we breathe out) can be the difference between life and death! In this activity you will learn the naming system for molecular compounds. Model 1 – Molecular Compounds Molecular Number of Atoms Number of Atoms in Name of Compound Formula of First Element Second Element ClF Chlorine monofluoride ClF5 1 5 Chlorine pentafluoride CO Carbon monoxide CO2 Carbon dioxide Cl2O Dichlorine monoxide PCl5 Phosphorus pentachloride N2O5 Dinitrogen pentoxide 1. Fill in the table to indicate the number of atoms of each type in the molecular formula. -

Midas Arsine
® Midas SENSOR CARTRIDGE SPECIFICATIONS Arsine (AsH3), Germane (GeH4) MIDAS-S-ASH, MIDAS-E-ASH Gas Measured Measured Arsine (AsH3) Cross Sensitivities Each Midas® sensor is potentially cross sensitive to other gases Cartridge Part Number MIDAS-S-ASH 1 year standard warranty MIDAS-E-ASH 2 year extended warranty and this may cause a gas reading when exposed to other gases than those originally designated. The table below presents typical Sensor Technology 3 electrode electrochemical cell readings that will be observed when a new sensor cartridge is Measuring Range (ppm) AsH 0 – 0.2ppm exposed to the cross sensitive gas (or a mixture of gases containing 3 the cross sensitive species). Minimum Alarm 1 Set Point 0.025ppm Gas / Vapor Chemical Concentration Reading Repeatability < ± 2% of measured value Formula applied (ppm) (ppm AsH3) Linearity < ± 10% of measured value Ammonia NH3 108 <0.1 Response Time t62.5 < 15 seconds Carbon Dioxide CO2 5,000 0 Sensor Cartridge Life Expectancy ≥ 24 months under typical application conditions Carbon Monoxide CO 85 0 Operating Temperature 0°C to + 40°C (32°C to 104°F) Chlorine CI2 TBD <-0.05 Effect of Temperature Diborane B2H6 0.1 0.05 Zero < ± 0.004 ppm / °C (0° to 40°C) Sensitivity < ± 0.9% of measured value / °C Disilane Si2H6 0.27 0.12 Operating Humidity (continuous) 10 – 95% rH non-condensing Germane GeH4 0.27 0.05 Effect of Humidity Hydrogen H2 3100 <-0.05 Zero Initial short term drift at abrupt rH change (< 0.001 ppm / % rH) Sensitivity < ± 0.2% of measured value / % rH Hydrogen Chloride HCI 7.9 0 -

Flow Injection Electrochemical Hydride Generation of Hydrogen Selenide on Lead Cathode: Critical Study of the Influence of Experimental Parameters
ANALYTICAL SCIENCES MARCH 2003, VOL. 19 367 2003 © The Japan Society for Analytical Chemistry Flow Injection Electrochemical Hydride Generation of Hydrogen Selenide on Lead Cathode: Critical Study of the Influence of Experimental Parameters Eduardo BOLEA, Francisco LABORDA,† and Juan R. CASTILLO Analytical Spectroscopy and Sensors Group, Department of Analytical Chemistry, University of Zaragoza, Zaragoza, Spain A flow injection system for the determination of selenium by electrochemical hydride generation and quartz tube atomic absorption spectrometry is described. The generator consists of an electrolytic flow-through cell with a concentric arrangement and a packed cathode made of particulated lead. The influences of sample flow rate, carrier gas flow rate and electrolysis current on the hydrogen selenide generation have been critically studied. Both sample flow rate and electrolysis current play important roles in the efficiency of the hydride generation process. A characteristic mass of 2.4 ng and a concentration detection limit of 17 µg l–1 were obtained for a sample volume of 420 µl. (Received January 22, 2002; Accepted September 25, 2002) The electrochemical generation of different hydrides on lead Introduction cathodes, the material that offers the highest hydrogen overpotential among those commonly used, has been studied by The formation of volatile covalent hydrides of a number of several authors.3–5,7–11 Sand3 and Thompson4,5 obtained elements (As, Bi, Ge, Sn, Sb, Se, Te and Pb) by reaction with generation efficiencies of 100%, whereas efficiencies of 86,11 sodium tetrahydroborate in acidic media has been widely used 928 and 60%11 were obtained for arsine, stibine and hydrogen for sample introduction in atomic spectrometry. -

Health-Based Reassessment of Administrative Occupational Exposure Limits
Gezondheidsraad Voorzitter Health Council of the Netherlands Aan de Staatssecretaris van Sociale Zaken en Werkgelegenheid Onderwerp : Aanbieding adviezen herevaluatie bestuurlijke MAC-waarden Uw kenmerk : ARBO/AMIL/97/00648 Ons kenmerk : U 2204/CB/mj/563-B5 Bijlagen : 1 Datum : 13 november 2001 Mijnheer de staatssecretaris, Op verzoek van uw ambtsvoorganger bied ik u hierbij 12 adviezen aan van een reeks over de gezondheidskundige basis van uit het buitenland overgenomen grenswaarden voor beroepsmatige blootstelling aan stoffen. Het verzoek om deze adviezen is in algemene zin vervat in brief nr ARBO/AMIL/97/00648 en in latere stadia door uw departement toegespitst op afzonderlijke stoffen. De adviezen zijn opgesteld door een daartoe door mij geformeerde internationale commissie van de Gezondheidsraad en beoordeeld door de Beraadsgroep Gezondheid en Omgeving. De beoogde reeks van in het Engels gestelde adviezen zal losbladig worden gepubliceerd onder ons publicatienummer 2000/15OSH en, conform de aan de Gezondheidsraad voorgelegde toespitsingen van de adviesaanvraag, betrekking hebben op 168 stoffen. Het u thans aangeboden tweede pakket bestaat uit de adviezen genummerd 2000/15OSH/018 tot en met 2000/15OSH/029, respectievelijk betrekking hebbend op: bornanon-2 (kamfer, synthetisch), chloortrifluoride, o-chloorstyreen, cyclohexylamine, dizwaveldecafluoride, hexafluoroaceton, keteen, pentachloornaftaleen, perchlorylfluoride, m-ftalodinitril, thionylchloride en trichloornaftaleen. Bij afronding van de werkzaamheden van de hierboven bedoelde -
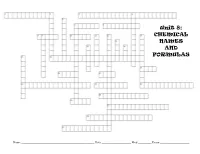
Unit 8: CHEMICAL NAMES and FORMULAS
Unit 8: CHEMICAL NAMES AND FORMULAS Name _________________________________________________________________ Date __________________________ Mod ____________ Exam __________________________ Across 28. These have various oxidation numbers 1. These have a 1+ oxidation number 29. The name for the compound of calcium and oxygen 3. Every compound always contains the same elements in the same proportions. Down 6. The type of compound containing only two elements 2. The number that tells how many atoms of an element are in a 7. Compounds form so that atoms can satisfy what rule? unit of the compound 8. ammonium chloride 4. A compound containing more than 2 different elements 10. The formula for chromium (III) and oxygen 5. A covalent bond wherein the electrons are shared equally 18. Name P4O10 among elements 19. silver oxide 6. The name for a compound of barium and hydroxide 20. A covalent bond wherein the electrons are not shared equally 9. These have a 1- oxidation number 21. The formula for beryllium and iodine 11. Nickel (I) carbonate 22. NH4OH 12. Name As2O5 23. A chemical combination of two or more elements having 13. The group which does not have oxidation numbers. different properties than the individual elements 14. Nona is the prefix for ___ in a covalent bond 24. Name Na2S 15. The prefix for 7 in a covalent bond 25. A single atom with a charge 16. The formula for calcium and oxygen 26. The prefix for 4 in a covalent bond 17. The ion ClO3- 27. The positive/negative number assigned to an element that shows its ability to combine in a compound Also for the exam: Be able to draw electron dot diagrams for various ionic bonds (brackets) and covalent bonds (dots). -

1. in an Ionic Compound of Formula Mmxn, the Metal (M) Occupies a Face-Centered Cubic Lattice and the Anions Occupy Four Positions Inside the Unit Cell
Name: __________________________ Date: _____________ 1. In an ionic compound of formula MmXn, the metal (M) occupies a face-centered cubic lattice and the anions occupy four positions inside the unit cell. Determine the formula of the compound. A) MX B) MX2 C) M2X D) MX3 E) M3X 2. Which one of the following is not a property of gases? A) particles in definite positions B) relatively low densities C) fills any container completely D) expand upon heating E) easily compressed 3. Give the systematic name for the following binary compound: S2F10 A) Disulfur decafluoride B) Sulfur fluoride C) Sulfur decafluoride D) Disulfur pentafluoride E) Disulfur fluoride 4. Which one of the following is not one of the hypotheses of Dalton's atomic theory? A) Atoms are composed of protons, neutrons, and electrons. B) All atoms of a given element are identical; the atoms of different elements are different and have different properties. C) Atoms of an element are not changed into different types of atoms by chemical reactions: atoms are neither created nor destroyed in chemical reactions. D) Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms. E) Each element is composed of extremely small particles called atoms. Page 1 5. What is the molar mass (g/mol) of sodium sulfite? A) 126.05 B) 142.05 C) 119.05 D) 103.05 E) 149.03 6. Ammonia reacts with oxygen gas to form nitric oxide (NO) and water vapour as follows: 4NH3 + 5O2 → 4NO + 6H2O What is the maximum amount of water that may be produced if 40.0 g NH3 and 50.0 g O2 are mixed and allowed to react? A) 1.88 mol B) 1.56 mol C) 3.52 mol D) 3.91 mol E) 2.35 mol 7. -

Biological Chemistry of Hydrogen Selenide
antioxidants Review Biological Chemistry of Hydrogen Selenide Kellye A. Cupp-Sutton † and Michael T. Ashby *,† Department of Chemistry and Biochemistry, University of Oklahoma, Norman, OK 73019, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-405-325-2924 † These authors contributed equally to this work. Academic Editors: Claus Jacob and Gregory Ian Giles Received: 18 October 2016; Accepted: 8 November 2016; Published: 22 November 2016 Abstract: There are no two main-group elements that exhibit more similar physical and chemical properties than sulfur and selenium. Nonetheless, Nature has deemed both essential for life and has found a way to exploit the subtle unique properties of selenium to include it in biochemistry despite its congener sulfur being 10,000 times more abundant. Selenium is more easily oxidized and it is kinetically more labile, so all selenium compounds could be considered to be “Reactive Selenium Compounds” relative to their sulfur analogues. What is furthermore remarkable is that one of the most reactive forms of selenium, hydrogen selenide (HSe− at physiologic pH), is proposed to be the starting point for the biosynthesis of selenium-containing molecules. This review contrasts the chemical properties of sulfur and selenium and critically assesses the role of hydrogen selenide in biological chemistry. Keywords: biological reactive selenium species; hydrogen selenide; selenocysteine; selenomethionine; selenosugars; selenophosphate; selenocyanate; selenophosphate synthetase thioredoxin reductase 1. Overview of Chalcogens in Biology Chalcogens are the chemical elements in group 16 of the periodic table. This group, which is also known as the oxygen family, consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive element polonium (Po). -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

Things to Be Done
DRAFT MAY 2003 ANNEX 1: CHEMICAL AGENTS 1. Introduction The large-scale use of toxic chemicals as weapons first became possible during the First World War (1914–1918) thanks to the growth of the chemical industry. More than 110 000 tonnes were disseminated over the battlefields, the greater part on the western front. Initially, the chemicals were used, not to cause casualties in the sense of putting enemy combatants out of action, but rather to harass. Though the sensory irritants used were powerful enough to disable those who were exposed to them, they served mainly to drive enemy combatants out of the trenches or other cover that protected them from conventional fire, or to disrupt enemy artillery or supplies. About 10% of the total tonnage of chemical warfare agents used during the war were chemicals of this type, namely lacrimators (tear gases), sternutators and vomiting agents. However, use of more lethal chemicals soon followed the introduction of disabling chemicals. In all, chemical agents caused some 1.3 million casualties, including 90 000 deaths. During the First World War, almost every known noxious chemical was screened for its potential as a weapon, and this process was repeated during the Second World War (1939–1945), when substantial stocks of chemical weapons were accumulated, although rarely used in military operations. Between the two world wars, a high proportion of all the new compounds that had been synthesized, or isolated from natural materials, were examined to determine their utility as lethal or disabling chemical weapons. After 1945, these systematic surveys continued, together with a search for novel agents based on advances in biochemistry, toxicology and pharmacology. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

2020 Emergency Response Guidebook
2020 A guidebook intended for use by first responders A guidebook intended for use by first responders during the initial phase of a transportation incident during the initial phase of a transportation incident involving hazardous materials/dangerous goods involving hazardous materials/dangerous goods EMERGENCY RESPONSE GUIDEBOOK THIS DOCUMENT SHOULD NOT BE USED TO DETERMINE COMPLIANCE WITH THE HAZARDOUS MATERIALS/ DANGEROUS GOODS REGULATIONS OR 2020 TO CREATE WORKER SAFETY DOCUMENTS EMERGENCY RESPONSE FOR SPECIFIC CHEMICALS GUIDEBOOK NOT FOR SALE This document is intended for distribution free of charge to Public Safety Organizations by the US Department of Transportation and Transport Canada. This copy may not be resold by commercial distributors. https://www.phmsa.dot.gov/hazmat https://www.tc.gc.ca/TDG http://www.sct.gob.mx SHIPPING PAPERS (DOCUMENTS) 24-HOUR EMERGENCY RESPONSE TELEPHONE NUMBERS For the purpose of this guidebook, shipping documents and shipping papers are synonymous. CANADA Shipping papers provide vital information regarding the hazardous materials/dangerous goods to 1. CANUTEC initiate protective actions. A consolidated version of the information found on shipping papers may 1-888-CANUTEC (226-8832) or 613-996-6666 * be found as follows: *666 (STAR 666) cellular (in Canada only) • Road – kept in the cab of a motor vehicle • Rail – kept in possession of a crew member UNITED STATES • Aviation – kept in possession of the pilot or aircraft employees • Marine – kept in a holder on the bridge of a vessel 1. CHEMTREC 1-800-424-9300 Information provided: (in the U.S., Canada and the U.S. Virgin Islands) • 4-digit identification number, UN or NA (go to yellow pages) For calls originating elsewhere: 703-527-3887 * • Proper shipping name (go to blue pages) • Hazard class or division number of material 2. -

3 • Molecules and Compounds
South Pasadena · AP Chemistry Name ____________________________________ Period ___ Date ___/___/___ 3 · Molecules and Compounds STUDY QUESTIONS and PROBLEMS 1. a. The structural formula for acetic acid is CH3CO2H. What is its empirical formula; what is its molecular formula? b. The molecular formula of acrylonitrile is C3H3N. Look up in the text, and draw, its structural formula. c. The molecular formula of aspartame (nutrasweet) is C14H18O5N2. Look up in the text, and draw, its structural formula. 2. The formulas for ethanol and ammonium nitrate are C2H5OH and NH4NO3. In what respects are these formulas and compounds different? 3. The molecular formula for both butanol and diethylether is C4H10O. Write structural formulas for both and show how they are different. Are any other structures possible? 4. Name the polyatomic ions: - - CH3CO2 HCO3 - 2- H2PO4 Cr2O7 2- - SO3 ClO 4 5. What are the formulas of the polyatomic ions: phosphate nitrite sulfate cyanide bisulfite chlorite 6. Write the ions present in the following salts and predict their formulas: potassium bromide calcium carbonate magnesium iodide lithium oxide aluminum sulfate ammonium chlorate beryllium phosphate 7. Name the following ionic salts: (NH4)2SO4 Co2(SO4)3 KHCO3 NiSO4 Ca(NO3)2 AlPO4 8. Name the following binary compounds of the nonmetals: CS2 SiCl4 SF6 GeH4 IF5 P4O10 N2H4 S4N4 PCl5 OF2 Cl2O7 IF7 9. What are the formulas for the following binary compounds? silicon dioxide phosphine boron trifluoride silicon carbide xenon tetroxide phosphorus tribromide dinitrogen pentoxide disulfur dichloride bromine trifluroide hydrogen selenide carbon tetrachloride 10. a. How many moles are present in 128 grams of sulfur dioxide? b.