Unrivalled Gas Detection. Ionscience.Com
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
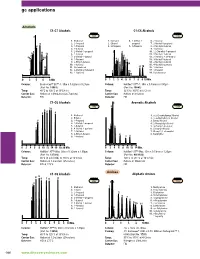
Gc Applications
gc applications Alcohols C1-C7 Alcohols C1-C6 Alcohols 1007 1255 1. Methanol 1. Methanol 4. 2-Methyl-2- 6. 2-Butanol 2. 2-Propanol 2. Ethanol propanol 7. 2-Methyl-1-propanol 3. 1-Propanol 3. 2-Propanol 5. 1-Propanol 8. 2-Methyl-2-butanol 4. 2-Butanol 9. 1-Butanol 5. 2-Methyl-1-propanol 10. 2,2-Dimethyl-1-propanol 6. 1-Butanol 11. 3-Methyl-2-butanol 7. 3-Methyl-1-butanol 12. 2-Pentanol + 3-Pentanol 8. 1-Pentanol 13. 3-Methyl-1-butanol 9. 2-Ethyl-1-butanol 14. 2-Methyl-1-butanol 10. 1-Hexanol 15. 4-Methyl-2-pentanol 11. Cyclohexanol 16. 1-Pentanol 12. 3-Methylcyclohexanol 17. 1-Hexanol 13. 1-Heptanol 18. Cyclohexanol å-IN Column: Econo-Cap™ EC™-1, 30m x 0.32mm x 0.25µm Column: Heliflex® AT™-1, 30m x 0.53mm x 5.00µm (Part No. 19651) (Part No. 16843) Temp: 40°C to 120°C at 10°C/min Temp: 35°C to 100°C at 5°C/min Carrier Gas: Helium at 1.09mL/min (26.7cm/sec) Carrier Gas: Helium at 3mL/min Detector: FID Detector: FID C1-C6 Alcohols Aromatic Alcohols 2596 1249 1. Methanol 1. A,A-Dimethylbenzyl Alcohol 2. Ethanol 2. DL-A-Methylbenzyl Alcohol 3. 1-Propanol 3. Benzyl Alcohol 4. 2-Methyl-1-propanol 4. 2-Phenylethyl Alcohol 5. 1-Butanol 5. 3-Phenyl-1-propanol 6. 4-Methyl-2-pentanol 6. Cinnamyl Alcohol 7. 1-Pentanol 7. Phenyl-1,2-ethanediol 8. 2-Ethyl-1-butanol 8. -

Catalytic Pyrolysis of Plastic Wastes for the Production of Liquid Fuels for Engines
Electronic Supplementary Material (ESI) for RSC Advances. This journal is © The Royal Society of Chemistry 2019 Supporting information for: Catalytic pyrolysis of plastic wastes for the production of liquid fuels for engines Supattra Budsaereechaia, Andrew J. Huntb and Yuvarat Ngernyen*a aDepartment of Chemical Engineering, Faculty of Engineering, Khon Kaen University, Khon Kaen, 40002, Thailand. E-mail:[email protected] bMaterials Chemistry Research Center, Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen, 40002, Thailand Fig. S1 The process for pelletization of catalyst PS PS+bentonite PP ) t e PP+bentonite s f f o % ( LDPE e c n a t t LDPE+bentonite s i m s n HDPE a r T HDPE+bentonite Gasohol 91 Diesel 4000 3500 3000 2500 2000 1500 1000 500 Wavenumber (cm-1) Fig. S2 FTIR spectra of oil from pyrolysis of plastic waste type. Table S1 Compounds in oils (%Area) from the pyrolysis of plastic wastes as detected by GCMS analysis PS PP LDPE HDPE Gasohol 91 Diesel Compound NC C Compound NC C Compound NC C Compound NC C 1- 0 0.15 Pentane 1.13 1.29 n-Hexane 0.71 0.73 n-Hexane 0.65 0.64 Butane, 2- Octane : 0.32 Tetradecene methyl- : 2.60 Toluene 7.93 7.56 Cyclohexane 2.28 2.51 1-Hexene 1.05 1.10 1-Hexene 1.15 1.16 Pentane : 1.95 Nonane : 0.83 Ethylbenzen 15.07 11.29 Heptane, 4- 1.81 1.68 Heptane 1.26 1.35 Heptane 1.22 1.23 Butane, 2,2- Decane : 1.34 e methyl- dimethyl- : 0.47 1-Tridecene 0 0.14 2,2-Dimethyl- 0.63 0 1-Heptene 1.37 1.46 1-Heptene 1.32 1.35 Pentane, -

Title Crystallization of Stereospecific Olefin Copolymers (Special Issue on Physical Chemistry) Author(S) Sakaguchi, Fumio; Kita
Crystallization of Stereospecific Olefin Copolymers (Special Title Issue on Physical Chemistry) Author(s) Sakaguchi, Fumio; Kitamaru, Ryozo; Tsuji, Waichiro Bulletin of the Institute for Chemical Research, Kyoto Citation University (1966), 44(4): 295-315 Issue Date 1966-10-31 URL http://hdl.handle.net/2433/76134 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University Crystallization of Stereospecifie Olefin Copolymers Fumio SAKAGUCHI,Ryozo KITAMARU and Waichiro TSUJI* (Tsuji Laboratory) Received August 13, 1966 The stereoregularity of isotactic poly(4-methyl-1-pentene) was characterized and isomorphism phenomena were examined for the copolymeric systems of 4-methyl-1-pentene with several olefins in order to study the crystallization phenomena in these olefin copoly- mers polymerized with stereospecific catalysts. The structural heterogeneity or the fine crystalline structure of poly(4-methyl-1-pentene) could be correlated with its molecular structure by viewing this stereoregular homopolymer as if it were a copolymer. Cocrystallization or isomorphism phenomenon was recognized for the copolymeric systems of 4-methyl-1-pentene with butene-1, pentene-1, decene-1 and 3-methyl-1-butene, while no evidence of the phenomenon was obtained for the copolymeric systems with styrene and propylene. The degree of the isomorphism of those copolymers was discussed with the informations on the crystalline phases obtained from the X-ray study, on the constitution of the copolymeric chains in the amorphous phases obtained from the viscoelastic studies and on the other thermodynamical properties of these systems. INTRODUCTION Many works have been made with regard to the homopolymerization of olefins with stereospecific catalysts, i. e. complex catalysts composed of the combination of organometallic compound and transitional metallic compound. -

Design for the Environment Toolkit: a Competitive Edge for the Future
Design for the Environment A Competitive Edge for the Future Toolkit Design for Environment Toolkit(DfE) Minnesota Office of Environmental Assistance Minnesota Technical Assistance Program (MnTAP) Table of Contents vAbout the Authors...2 vAcknowledgments...3 vPreface...4 vPart One: Introduction to Design for the Environment (DfE)...6 What is DfE?...6 Why DfE?...10 Success in DfE...12 Implementing DfE...13 vPart Two: DfE Matrices and Questions...17 Explanation of the DfE Matrix System..17 Figure: Product DfE Matrix...18 Instructions For Using the DfE Matrix...19 Interpretation of Results...28 Matrix Questions...20 Definitions...30 vPart Three: Reference Information...32 Index of Hazardous Chemical Pollutants (alphabetical listing)...32 Plastics Environmental Risk Information...57 Figure: Recycling Rates and Commodity Price Information....58 Index of Climate Altering Chemicals...59 Polymers....61 Degradable Polymers - Vendor List...62 Part/Materials Suppliers Environmental Survey...65 Additional Resources...66 Works Cited....68 Summary of References...69 Other Vendor Lists Checklist of Printed Resources for Minnesota Businesses Outlets for Industrial Scrap Pallets Alternative Solvent Degreasers Manufacturers of Aqueous Cleaning Equipment Aqueous and Semi-Aqueous Cleaners for Metal Part Degreasing Safer Stripping and Cleaning Chemicals for Coatings and Polymers Page 1 About the Authors Jeremy M. Yarwood - Research Specialist, Minnesota Technical Assistance Program (MnTAP) Mr. Yarwood has previously worked with MnTAP advising Minnesota companies in developing environmentally responsible processes. He received his B.S. Civil (Environmental) Engineering summa cum laude from the University of Minnesota. Mr. Yarwood is currently a graduate fellow in the Department of Civil Engineering at the University of Minnesota and plans to obtain a M.S. -

Highly Efficient Olefin Isomerization Catalyzed by Metal Hydrides Derives from Dirhodium(Ii) Carboxylates and Catecholborane
HIGHLY EFFICIENT OLEFIN ISOMERIZATION CATALYZED BY METAL HYDRIDES DERIVES FROM DIRHODIUM(II) CARBOXYLATES AND CATECHOLBORANE Gene A. Devora and Michael P. DoyleL' * Department of Chemistry, Trinity University, San Antonio, Texas 78212, USA Abstract. Dirhodium(ll) tetraacetate in combination with catecholborane catalyzes the iso- merization of alkenes and dienes. Effective isomerization occurs at 80-135°C with the use of only 0.1 mol % rhodium acetate. With 2-methyl-1,5-hexadiene the disubstituted double bond is prefer- entially isomerized. In addition, hydrogen transfer hydrogenation occurs with 1,4-cyclohexadienes. The mechanism of these reactions is proposed to involve organoborane addition across a Rh-0 bond which activates the catalyst for isomerization and hydrogenation. INTRODUCTION Catalytic isomerization of alkenes is a characteristic transformation of transition metal hy- drides that often accompanies hydrogenation1 and is one of the most thoroughly studied catalytic reactions.2"4 Compounds of cobalt, nickel, palladium, platinum, rhodium, and ruthenium are effective,2 but other transition metal compounds have also been employed for catalytic isomeriza- tions.2"4 Although the nature of this transformation is dependent on the catalyst, selectivity for alkene isomerization generally favors reactions with monosubstituted ethylenes over di- and tri-sub- stituted ethylenes. In the course of our investigations of the catalytic effectiveness of dirhodium(ll) tetrakis(carboxylates) we have uncovered a useful methodology for the generation of rhodium hydride species that, as we now report, are surprisingly effective for the isomerization of alkenes as well as for hydrogen transfer hydrogenation. MATERIALS AND METHODS Reactions were performed in a round bottom flask equipped with a screw cap that was fitted with a septum for convenient withdrawal of aliquots. -

A Practical and Stereoselective Synthesis of Cinnamyl Alcohols Bearing A-Cyano Or A-Ester Functional Group from Baylis-Hillman Adducts
Notes Bull. Korean Chem. Soc. 2004, Vol. 25, No. 3 413 A Practical and Stereoselective Synthesis of Cinnamyl Alcohols Bearing a-Cyano or a-Ester Functional Group from Baylis-Hillman Adducts Ka Young Lee, Saravanan GowriSankar, and Jae Nyoung Kim Department of Chemistry and Institute of Basic Science, Chonnam National University, Gwangju 500-757, Korea Received December 18, 2003 Key Words : Cinnamyl alcohols, Baylis-Hillman adducts, Sulfuric acid, Acetic anhydride Cinnamyl alcohols bearing ester, acid, or nitrile functional Hillman alcohol into the primary acetate or eventually to group at the 2-position are very useful synthons for the primary alcohol (Scheme 1 and Table 1). synthesis of various biologically important compounds.1 The reaction of Baylis-Hillman adducts with acetic Synthesis of cinnamyl alcohol derivatives from the Baylis- anhydride (1.5 equiv.) in the presence of sulfuric acid (10 Hillman adducts has been reported by us and other groups.1-4 mol%) gave the corresponding rearranged cinnamyl acetates The conversion can be carried out directly by using 20% quantitatively in dichloromethane at room temperature aqueous sulfuric acid (for the Baylis-Hillman adducts within 30 min (Actually, the cinnamyl acetate derivatives derived from acrylonitrile)2 or trifluoroacetic acid (for the could be isolated in 92-95% yields.). In order to obtain the Baylis-Hillman adducts derived from acrylates).3 Recently, corresponding cinnamyl alcohols in a one-pot we tried some Basavaiah and coworkers have reported the two-step, one- reaction conditions for the next partial hydrolysis. Addition pot conversion method: TMSOTf-assisted conversion of of water to the reaction mixture (two-phase system) afforded Baylis-Hillman alcohol into the corresponding primary the hydrolyzed cinnamyl alcohols in trace amounts. -

September 17, 2007
Pre-Feasibility Report M/s. Neogen Chemicals Ltd. 1 1. Introduction M/s. Neogen Chemicals Ltd. is a new unit located at Plot No. Z/96/B SEZ Dahej, District: Bharuch, Gujarat. Now, the unit proposes to manufacture different type of synthetic organic dyes and pesticide products at above sited address. 2. Cost of Project Cost of existing project is 55 crore &, out of which 5 crore will be used for Environment Management System. 3. Production Capacity Production capacity is prescribe below: List of Products Sr. Name of Products Quantity No. (MT/Year) (MT/month) 1 Bromination and Chlorination of Alcohols 1.1. Ethyl Bromide 3500 291.67 1.2. n-Propyl Bromide 1.3. Iso Propyl Bromide 1.4. n-Butyl Bromide 1.5. Iso Butyl Bromide 1.6. Sec-Butyl Bromide 1.7. n-Hexyl Bromide 1.8. n-Heptyl Bromide 1.9. n-Octyl Bromide 1.10. n-Decyl Bromide 1.11. Lauryl Bromide 1.12. Cetyl Bromide 1.13. Myristyl Bromide 1.14. Stearyl Bromide 1.15. 1,2 Dibromo Ethane 1.16. 1,3 Dibromo Propane 1.17. 1,4 Dibromo Butane 1.18. 1,5 Dibromo pentane M/s. Neogen Chemicals Ltd. 2 Sr. Name of Products Quantity No. (MT/Year) (MT/month) 1.19. 1,6 Dibromo Hexane 1.20. 1 Chloro 2 Ethyl Hexane 1.21. 6 Chloro 1 Hexanol 1.22. 3 Chloro Propanol 1.23. 1,6 Dichloro Hexane 1.24. Cyclo Propyl Methyl Bromide 1.25. Cyclo Pentyl Bromide 1.26. Cyclo Pentyl Chloride 2. Bromination of Organic Acids and Esterification thereof 2.1. -

Naming Molecular Compounds General Instructions: Please Do the Activities for Each Day As Indicated
Teacher Name: Dwight Lillie Student Name: ________________________ Class: ELL Chemistry Period: Per 4 Assignment: Assignment week 2 Due: Friday, 5/8 Naming Molecular Compounds General Instructions: Please do the activities for each day as indicated. Any additional paper needed please attach. Submitted Work: 1) Completed packet. Questions: Please send email to your instructor and/or attend published virtual office hours. Schedule: Date Activity Monday (4/27) Read Sections 9.3, 9.5 in your textbook. Tuesday (4/28) Read and work through questions 1-9 Wednesday (4/29) Read and work through questions 10-14 Thursday (4/30) Read and work through questions 14-18 Friday (5/31) Read and work through questions 19-21 How are the chemical formula and name of a molecular compound related? Why? When you began chemistry class this year, you probably already knew that the chemical formula for carbon dioxide was CO2. Today you will find out why CO2 is named that way. Naming chemical compounds correctly is of paramount importance. The slight difference between the names carbon monoxide (CO, a poisonous, deadly gas) and carbon dioxide (CO2, a greenhouse gas that we exhale when we breathe out) can be the difference between life and death! In this activity you will learn the naming system for molecular compounds. Model 1 – Molecular Compounds Molecular Number of Atoms Number of Atoms in Name of Compound Formula of First Element Second Element ClF Chlorine monofluoride ClF5 1 5 Chlorine pentafluoride CO Carbon monoxide CO2 Carbon dioxide Cl2O Dichlorine monoxide PCl5 Phosphorus pentachloride N2O5 Dinitrogen pentoxide 1. Fill in the table to indicate the number of atoms of each type in the molecular formula. -

Food and Drug Administration, HHS § 172.515
Food and Drug Administration, HHS § 172.515 Common name Scientific name Limitations Tolu ................................................................ Myroxylon balsamum (L.) Harms. Turpentine ...................................................... Pinus palustris Mill. and other Pinus spp. which yield terpene oils exclusively. Valerian rhizome and roots ............................ Valeriana officinalis L. Veronica ......................................................... Veronica officinalis L .................................................. Do. Vervain, European ......................................... Verbena officinalis L ................................................... Do. Vetiver ............................................................ Vetiveria zizanioides Stapf ......................................... Do. Violet, Swiss ................................................... Viola calcarata L. Walnut husks (hulls), leaves, and green nuts Juglans nigra L. or J. regia L. Woodruff, sweet ............................................. Asperula odorata L ..................................................... In alcoholic beverages only Yarrow ............................................................ Achillea millefolium L .................................................. In beverages only; fin- ished beverage thujone free1 Yerba santa .................................................... Eriodictyon californicum (Hook, et Arn.) Torr. Yucca, Joshua-tree ........................................ Yucca brevifolia Engelm. Yucca, -

Nomination Background: Methyl Trans
' 7/?1 Methyl styryl ketone 122-57-6 NTP NOMINATION HISTORY AND REVIEW A. Nomination History 1. Nomination Source: NCI 2. Recommendations: -Comparative toxicity studies with methyl vinyl ketone -Metabolism -Carcinogenicity 3. Rationale/Remarks: -Potential for widespread human exposure -Nominated as a result of a class study of a,~-unsaturated ketones -Flavoring and fragrance additive -Natural product -Environmental pollutant -Mutagenic in Salmonella 4. Priority: -High for toxicity studies -Moderate for carcinogenicity s. Date of Nomination: July 1994 B. Interagency Committee for Chemical Evaluation and Coordination Review 1. Date of Review: 2. Recommendations: 3. Priority: 4. NTP Chemical Selection Principles: 5. Rationale/Remarks: 122-57-6 Methyl styryl ketone SUMMARY OF DATA FOR CHEMICAL SELECTION CHEMICAL IDENTIFICATION CAS Registry Number: 122-57-6 Chemical Abstracts Name: 3-Buten-2-one, 4-phenyl- (SCI, 9CI) Svnonvms and Trade Names: Acetocinnamone; benzalacetone; benzylideneacetone; methyl 2 phenylvinyl ketone; methyl styryl ketone; methyl ~-styryl ketone; 4 phenylbutenone; 4-phenyl-3-butene-2-one; 2-phenylvinyl methyl ketone; styryl methyl ketone; MSK FEMA Number: 2881 Related isomers CAS Registry Number: 937-53-1 Chemica] Abstracts Name: 3-Buten-2-one, 4-phenyl-, (Z)- (SCI, 9CI) Synonvms and Trade Names: cis -4-Phenyl-3-buten-2-one; cis -benzalacetone; cis benzylideneacetone; cis-methyl styryl ketone CAS Registry Number: 1896-62-4 Chemical Abstracts Name: 3-Buten-2-one, 4-phenyl-, (E)- (SCI, 9CI) Synonyms and Trade Names: trans-4-Phenyl-3-buten-2-one; trans-benzalacetone; trans benzylideneacetone; trans-methyl styryl ketone; TPBO Structure. Molecular Formula. and Molecular WeiKbt 0 II H•CHCCH3 Mol. wt.: 146.19 Chemical and Phvsical Properties (from Aldrich Chemical Co., 1993, 1994; Budavari, 1989) Description: White to yellow solid with a sweet, pungent, creamy, floral odor Prepared for NCI by Technical Resources, Inc. -

Functional Expression of a Novel Methanol-Stable Esterase From
Cai et al. BMC Biotechnology (2020) 20:36 https://doi.org/10.1186/s12896-020-00622-1 RESEARCH ARTICLE Open Access Functional expression of a novel methanol- stable esterase from Geobacillus subterraneus DSM13552 for biocatalytic synthesis of cinnamyl acetate in a solvent- free system Xianghai Cai1†, Lin Lin2,3†, Yaling Shen1, Wei Wei1* and Dong-zhi Wei1 Abstract Background: Esterases are widely distributed in nature and have important applications in medical, industrial and physiological. Recently, the increased demand for flavor esters has prompted the search of catalysts like lipases and esterases. Esterases from thermophiles also show thermal stability at elevated temperatures and have become enzymes of special interest in biotechnological applications. Although most of esterases catalyzed reactions are carried out in toxic and inflammable organic solvents, the solvent-free system owning many advantages such as low cost and easy downstream processing. Results: The gene estGSU753 from Geobacillus subterraneus DSM13552 was cloned, sequenced and overexpressed into Escherichia coli BL21 (DE3). The novel gene has an open reading frame of 753 bp and encodes 250-amino-acid esterase (EstGSU753). The sequence analysis showed that the protein contains a catalytic triad formed by Ser97, Asp196 and His226, and the Ser of the active site is located in the conserved motif Gly95-X-Ser97-X-Gly99 included in most esterases and lipases. The protein catalyzed the hydrolysis of pNP-esters of different acyl chain lengths, and the enzyme specific activity was 70 U/mg with the optimum substrate pNP-caprylate. The optimum pH and temperature of the recombinant enzyme were 8.0 and 60 °C respectively. -

Health-Based Reassessment of Administrative Occupational Exposure Limits
Gezondheidsraad Voorzitter Health Council of the Netherlands Aan de Staatssecretaris van Sociale Zaken en Werkgelegenheid Onderwerp : Aanbieding adviezen herevaluatie bestuurlijke MAC-waarden Uw kenmerk : ARBO/AMIL/97/00648 Ons kenmerk : U 2204/CB/mj/563-B5 Bijlagen : 1 Datum : 13 november 2001 Mijnheer de staatssecretaris, Op verzoek van uw ambtsvoorganger bied ik u hierbij 12 adviezen aan van een reeks over de gezondheidskundige basis van uit het buitenland overgenomen grenswaarden voor beroepsmatige blootstelling aan stoffen. Het verzoek om deze adviezen is in algemene zin vervat in brief nr ARBO/AMIL/97/00648 en in latere stadia door uw departement toegespitst op afzonderlijke stoffen. De adviezen zijn opgesteld door een daartoe door mij geformeerde internationale commissie van de Gezondheidsraad en beoordeeld door de Beraadsgroep Gezondheid en Omgeving. De beoogde reeks van in het Engels gestelde adviezen zal losbladig worden gepubliceerd onder ons publicatienummer 2000/15OSH en, conform de aan de Gezondheidsraad voorgelegde toespitsingen van de adviesaanvraag, betrekking hebben op 168 stoffen. Het u thans aangeboden tweede pakket bestaat uit de adviezen genummerd 2000/15OSH/018 tot en met 2000/15OSH/029, respectievelijk betrekking hebbend op: bornanon-2 (kamfer, synthetisch), chloortrifluoride, o-chloorstyreen, cyclohexylamine, dizwaveldecafluoride, hexafluoroaceton, keteen, pentachloornaftaleen, perchlorylfluoride, m-ftalodinitril, thionylchloride en trichloornaftaleen. Bij afronding van de werkzaamheden van de hierboven bedoelde