Agenda 12/9/19 Period 7
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemicals, Rare Chemicals, Reagents & Solvents
Annual Procurement Plan for the Year 2021-22 Consumable List 1 Chemicals, Rare Chemicals, Reagents & Solvents Sl. No. Generic Name Specification Quantity Approx Cost (Rs.) 1. HEPES Molecular biology 1kg 80000/‐ grade 2. MES hydrate Molecular biology 1kg 34000/‐ grade 3. Imidazole Molecular biology 1kg 28000/‐ grade 4. BIS‐TRIS Molecular biology 500g 44000/‐ grade 5. Agarose Type I Molecular biology 1kg 11900/‐ grade 6. CAPS Molecular biology 100g 8000/‐ grade 7. Trizma Base Molecular biology 1kg 31000/‐ grade 8. Glycerol Molecular biology 2.5L*4 8000/‐ grade 9. Ethanol Molecular biology 500ml*10 8000/‐ grade 10. NaCl Molecular biology 1kg 6000/‐ grade 11. Tris Buffer Molecular biology 1 kg 10000/‐ grade 12. Ethyl acetate Molecular biology 500ml*5 6500/‐ grade 13. Acetic acid 25L 11000/‐ 14. Methanol 25L 8000/‐ 15. Urea Molecular biology 1kg 2000/‐ grade 16. Agar Powder, Bacteriological 500g 8000/‐ 17. Trizma Hydrochloride Molecular biology 1kg 15000/‐ grade 18. Acrylamide Molecular biology 500g 4000/‐ grade 19. Phenol Molecular biology 500ml 5000/‐ grade 20. Luria Bertani Broth 2.5kg * 4 90,000 21. Acetone 2.5L 1500/‐ 22. Ni‐NTA beads 100ml 57000/‐ 23. Ammonium persulphate Molecular biology 100g 2000/‐ grade 24. Ampicillin Sodium Molecular biology 100 g 13000/‐ grade 25. Kanaymycin Monosulfate Molecular biology 100g 60000/‐ grade 26. Choramphenicol Molecular biology 100g 40000/‐ grade 27. Isopropyl β‐ d‐1‐thiogalactopyranoside Molecular biology 100g 35000/‐ grade 28. Coomassie Brilliant Blue R‐250 Molecular biology 25g 10,000/‐ grade 29. SDS Molecular biology 100g 2500/‐ grade 30. Isopropanol 25 L 15000/‐ 31. Crystallization screen JCSG‐Plus 1 unit/box 50000/‐ 32. -

Synthetic Routes to Bromo-Terminated Phosphonate Films and Alkynyl Pyridine Compounds for Click Coupling
University of Mary Washington Eagle Scholar Student Research Submissions Spring 5-7-2018 Synthetic Routes to Bromo-Terminated Phosphonate Films and Alkynyl Pyridine Compounds for Click Coupling Poornima Sunder Follow this and additional works at: https://scholar.umw.edu/student_research Part of the Biochemistry Commons Recommended Citation Sunder, Poornima, "Synthetic Routes to Bromo-Terminated Phosphonate Films and Alkynyl Pyridine Compounds for Click Coupling" (2018). Student Research Submissions. 226. https://scholar.umw.edu/student_research/226 This Honors Project is brought to you for free and open access by Eagle Scholar. It has been accepted for inclusion in Student Research Submissions by an authorized administrator of Eagle Scholar. For more information, please contact [email protected]. Synthetic Routes to Bromo-Terminated Phosphonate Films and Alkynyl Pyridine Compounds for Click Coupling Poornima Rachel Sunder Thesis submitted to the faculty of University of Mary Washington in partial fulfillment of the requirements for graduation with Honors in Chemistry (2018) ABSTRACT Click reactions are a highly versatile class of reactions that produce a diverse range of products. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) click reactions require an azide and a terminal alkyne and produce a coupled product that is “clicked” through a triazole ring that can have a variety of substituents. In this work, bromo-terminated phosphonate films on copper oxide surfaces were explored as the platform for click coupling, as the terminal azide needed for the reaction can be generated through an in situ SN2 reaction with a terminal bromo group. The reactions were characterized using model reactions in solution before being conducted on modified copper oxide surfaces. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Synthesis and Antiplasmodial Activity Testing of (1)-N-(4-Methoxybenzyl)-1,10-Phenanthrolinium Bromide
Indo. J. Chem., 2007, 7 (2), 197-201 197 SYNTHESIS AND ANTIPLASMODIAL ACTIVITY TESTING OF (1)-N-(4-METHOXYBENZYL)-1,10-PHENANTHROLINIUM BROMIDE 1,* 2 2 3 3 Ruslin Hadanu , Sabirin Mastjeh , Jumina , Mustofa , Mahardika Agus Widjayanti and Eti Nurwening Sholikhah3 1Department of Chemistry, Pattimura University, Ambon, Indonesia. 2Laboratorium of Organic Chemistry, Department of Chemistry, Universitas Gadjah Mada, Yogyakarta, Indonesia 55281. 3Medical Faculty, Universitas Gadjah Mada, Yogyakarta, Indonesia 55281. Received 7 April 2007; Accepted 25 May 2007 ABSTRACT Synthesis of (1)-N-(4-methoxybenzyl)-1,10-phenanthroline bromide from 1,10-phenanthroline monohydrate and 4-methoxybenzaldehyde as starting material and evaluation of its antiplasmodial activities have been carried out. The 4-methoxybenzyl alcohol was prepared from 4-methoxy-benzaldehyde using sodium borohydride (NaBH4) reagent and ethanol absolute solution. The mixture was refluxed for 3 h. To yield colorless dilution compound with 90.41 % in efficiency. Furthermore, bromination of 4-methoxybenzyl alcohol with phosphorus bromide (PBr3) was conducted by refluxing for 3 h. The product of this reaction was yellow liquid of 4-methoxybenzyl bromide, 79.03% yield and 95.34 % purity. The final step of reaction was benzylation of 1,10-phenanthroline monohydrate with 4- methoxybenzyl bromide reagent. It was conducted by refluxing in aceton for 8 h at 55 oC. The yield of the reaction was (1)-N-(4-methoxybenzyl)-1,10-phenanthroline bromide (77.63%). It is pink solid form, and its melting point is 192-193 oC. Identification of the product was carried out by means of GC-MS, IR and 1H-NMR spectrometers. The in vitro antiplasmodial activity on chloroquine-resistant Plasmodium falciparum FCR-3 strain and chloroquine sensitive P. -

Alphabetical Index of Substances and Articles
ALPHABETICAL INDEX OF SUBSTANCES AND ARTICLES - 355 - NOTES TO THE INDEX 1. This index is an alphabetical list of the substances and articles which are listed in numerical order in the Dangerous Goods List in Chapter 3.2. 2. For the purpose of determining the alphabetical order the following information has been ignored even when it forms part of the proper shipping name: numbers; Greek letters; the abbreviations “sec” and “tert”; and the letters “N” (nitrogen), “n” (normal), “o” (ortho) “m” (meta), “p” (para) and “N.O.S.” (not otherwise specified). 3. The name of a substance or article in block capital letters indicates a proper shipping name. 4. The name of a substance or article in block capital letters followed by the word “see” indicates an alternative proper shipping name or part of a proper shipping name (except for PCBs). 5. An entry in lower case letters followed by the word “see” indicates that the entry is not a proper shipping name; it is a synonym. 6. Where an entry is partly in block capital letters and partly in lower case letters, the latter part is considered not to be part of the proper shipping name. 7. A proper shipping name may be used in the singular or plural, as appropriate, for the purposes of documentation and package marking. - 356 - INDEX Name and description Class UN No. Name and description Class UN No. Accumulators, electric, see 4.3 3292 Acid mixture, nitrating acid, see 8 1796 8 2794 8 2795 Acid mixture, spent, nitrating acid, see 8 1826 8 2800 8 3028 Acraldehyde, inhibited, see 6.1 1092 ACETAL 3 1088 -
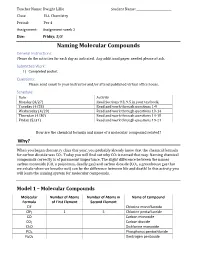
Naming Molecular Compounds General Instructions: Please Do the Activities for Each Day As Indicated
Teacher Name: Dwight Lillie Student Name: ________________________ Class: ELL Chemistry Period: Per 4 Assignment: Assignment week 2 Due: Friday, 5/8 Naming Molecular Compounds General Instructions: Please do the activities for each day as indicated. Any additional paper needed please attach. Submitted Work: 1) Completed packet. Questions: Please send email to your instructor and/or attend published virtual office hours. Schedule: Date Activity Monday (4/27) Read Sections 9.3, 9.5 in your textbook. Tuesday (4/28) Read and work through questions 1-9 Wednesday (4/29) Read and work through questions 10-14 Thursday (4/30) Read and work through questions 14-18 Friday (5/31) Read and work through questions 19-21 How are the chemical formula and name of a molecular compound related? Why? When you began chemistry class this year, you probably already knew that the chemical formula for carbon dioxide was CO2. Today you will find out why CO2 is named that way. Naming chemical compounds correctly is of paramount importance. The slight difference between the names carbon monoxide (CO, a poisonous, deadly gas) and carbon dioxide (CO2, a greenhouse gas that we exhale when we breathe out) can be the difference between life and death! In this activity you will learn the naming system for molecular compounds. Model 1 – Molecular Compounds Molecular Number of Atoms Number of Atoms in Name of Compound Formula of First Element Second Element ClF Chlorine monofluoride ClF5 1 5 Chlorine pentafluoride CO Carbon monoxide CO2 Carbon dioxide Cl2O Dichlorine monoxide PCl5 Phosphorus pentachloride N2O5 Dinitrogen pentoxide 1. Fill in the table to indicate the number of atoms of each type in the molecular formula. -

United States Patent Office Patented Jan
3,119,666 United States Patent Office Patented Jan. 28, 1964 1. 2 solvent is immaterial, since in practical operation, I re 3,119,666 use the solvent for the reaction after the suspended phos METHOS FOR THE PREPARATION OF phorus pentabromide is removed therefrom by filtration PHOSPHORUS PENTABROMIDE or by centrifuging after the reaction is completed. There Richard C. Nametz, St. Louis, Mich., assignor to fore, upon the first use of the solvent it becomes satu Michigan Chemical Corporation, St. Louis, Mich., a rated with the small amount of phosphorus pentabromide corporation of Michigan which it will dissolve, and will dissolve no more of the No Drawing. Fied Nov. 12, 1957, Ser. No. 695,548 product upon reuse. 12 Claims. (C. 23-205) In this method, I utilize substantially equimolar quan This invention relates to an improved method for the O tities of bromide and of phosphorus tribromide, together production of phosphorus pentabromide. with an amount of the co-solvent for bromine and phos Phosphorus pentabromide (phosphoric bromide) is a phorus tribromide relative to the quantities of the re known compound having a melting point above 100 C., actants used which is within the range which will suspend at which temperature it decomposes to form phosphorus the phosphorus pentabromide as a slurry which can be tribromide with the evolution of bromine. Due to its 5 readily stirred and which at the end of the reaction can heat sensitivity, phosphorus pentabromide cannot be puri be readily filtered, but which does not provide an un wieldly bulk of material to handle. -

Operation Permit Application
Un; iy^\ tea 0 9 o Operation Permit Application Located at: 2002 North Orient Road Tampa, Florida 33619 (813) 623-5302 o Training Program TRAINING PROGRAM for Universal Waste & Transit Orient Road Tampa, Florida m ^^^^ HAZARDOUS WAb 1 P.ER^AlTTlNG TRAINING PROGRAM MASTER INDEX CHAPTER 1: Introduction Tab A CHAPTER 2: General Safety Manual Tab B CHAPTER 3: Protective Clothing Guide Tab C CHAPTER 4: Respiratory Training Program Tab D APPENDIX 1: Respiratory Training Program II Tab E CHAPTER 5: Basic Emergency Training Guide Tab F CHAPTER 6: Facility Operations Manual Tab G CHAPTER 7: Land Ban Certificates Tab H CHAPTER 8: Employee Certification Statement Tab. I CHAPTER ONE INTRODUCTION prepared by Universal Waste & Transit Orient Road Tampa Florida Introducti on STORAGE/TREATMENT PERSONNEL TRAINING PROGRAM All personnel involved in any handling, transportation, storage or treatment of hazardous wastes are required to start the enclosed training program within one-week after the initiation of employment at Universal Waste & Transit. This training program includes the following: Safety Equipment Personnel Protective Equipment First Aid & CPR Waste Handling Procedures Release Prevention & Response Decontamination Procedures Facility Operations Facility Maintenance Transportation Requirements Recordkeeping We highly recommend that all personnel involved in the handling, transportation, storage or treatment of hazardous wastes actively pursue additional technical courses at either the University of South Florida, or Tampa Junior College. Recommended courses would include general chemistry; analytical chemistry; environmental chemistry; toxicology; and additional safety and health related topics. Universal Waste & Transit will pay all registration, tuition and book fees for any courses which are job related. The only requirement is the successful completion of that course. -

Prohibited and Restricted Chemical List
School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List PROHIBITED AND RESTRICTED CHEMICAL LIST Introduction After incidents of laboratory chemical contamination at several schools, DCPS, The American Association for the Advancement of Science (AAAS) and DC Fire and Emergency Management Services developed an aggressive program for chemical control to eliminate student and staff exposure to potential hazardous chemicals. Based upon this program, all principals are required to conduct a complete yearly inventory of all chemicals located at each school building to identify for the removal and disposal of any prohibited/banned chemicals. Prohibited chemicals are those that pose an inherent, immediate, and potentially life- threatening risk, injury, or impairment due to toxicity or other chemical properties to students, staff, or other occupants of the school. These chemicals are prohibited from use and/or storage at the school, and the school is prohibited from purchasing or accepting donations of such chemicals. Restricted chemicals are chemicals that are restricted by use and/or quantities. If restricted chemicals are present at the school, each storage location must be addressed in the school's written emergency plan. Also, plan maps must clearly denote the storage locations of these chemicals. Restricted chemicals—demonstration use only are a subclass in the Restricted chemicals list that are limited to instructor demonstration. Students may not participate in handling or preparation of restricted chemicals as part of a demonstration. If Restricted chemicals—demonstration use only are present at the school, each storage location must be addressed in the school's written emergency plan. Section 7: Appendices – October 2009 37 School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List Following is a table of chemicals that are Prohibited—banned, Restricted—academic curriculum use, and Restricted—demonstration use only. -

Health-Based Reassessment of Administrative Occupational Exposure Limits
Gezondheidsraad Voorzitter Health Council of the Netherlands Aan de Staatssecretaris van Sociale Zaken en Werkgelegenheid Onderwerp : Aanbieding adviezen herevaluatie bestuurlijke MAC-waarden Uw kenmerk : ARBO/AMIL/97/00648 Ons kenmerk : U 2204/CB/mj/563-B5 Bijlagen : 1 Datum : 13 november 2001 Mijnheer de staatssecretaris, Op verzoek van uw ambtsvoorganger bied ik u hierbij 12 adviezen aan van een reeks over de gezondheidskundige basis van uit het buitenland overgenomen grenswaarden voor beroepsmatige blootstelling aan stoffen. Het verzoek om deze adviezen is in algemene zin vervat in brief nr ARBO/AMIL/97/00648 en in latere stadia door uw departement toegespitst op afzonderlijke stoffen. De adviezen zijn opgesteld door een daartoe door mij geformeerde internationale commissie van de Gezondheidsraad en beoordeeld door de Beraadsgroep Gezondheid en Omgeving. De beoogde reeks van in het Engels gestelde adviezen zal losbladig worden gepubliceerd onder ons publicatienummer 2000/15OSH en, conform de aan de Gezondheidsraad voorgelegde toespitsingen van de adviesaanvraag, betrekking hebben op 168 stoffen. Het u thans aangeboden tweede pakket bestaat uit de adviezen genummerd 2000/15OSH/018 tot en met 2000/15OSH/029, respectievelijk betrekking hebbend op: bornanon-2 (kamfer, synthetisch), chloortrifluoride, o-chloorstyreen, cyclohexylamine, dizwaveldecafluoride, hexafluoroaceton, keteen, pentachloornaftaleen, perchlorylfluoride, m-ftalodinitril, thionylchloride en trichloornaftaleen. Bij afronding van de werkzaamheden van de hierboven bedoelde -
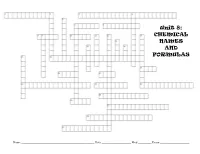
Unit 8: CHEMICAL NAMES and FORMULAS
Unit 8: CHEMICAL NAMES AND FORMULAS Name _________________________________________________________________ Date __________________________ Mod ____________ Exam __________________________ Across 28. These have various oxidation numbers 1. These have a 1+ oxidation number 29. The name for the compound of calcium and oxygen 3. Every compound always contains the same elements in the same proportions. Down 6. The type of compound containing only two elements 2. The number that tells how many atoms of an element are in a 7. Compounds form so that atoms can satisfy what rule? unit of the compound 8. ammonium chloride 4. A compound containing more than 2 different elements 10. The formula for chromium (III) and oxygen 5. A covalent bond wherein the electrons are shared equally 18. Name P4O10 among elements 19. silver oxide 6. The name for a compound of barium and hydroxide 20. A covalent bond wherein the electrons are not shared equally 9. These have a 1- oxidation number 21. The formula for beryllium and iodine 11. Nickel (I) carbonate 22. NH4OH 12. Name As2O5 23. A chemical combination of two or more elements having 13. The group which does not have oxidation numbers. different properties than the individual elements 14. Nona is the prefix for ___ in a covalent bond 24. Name Na2S 15. The prefix for 7 in a covalent bond 25. A single atom with a charge 16. The formula for calcium and oxygen 26. The prefix for 4 in a covalent bond 17. The ion ClO3- 27. The positive/negative number assigned to an element that shows its ability to combine in a compound Also for the exam: Be able to draw electron dot diagrams for various ionic bonds (brackets) and covalent bonds (dots). -

1. in an Ionic Compound of Formula Mmxn, the Metal (M) Occupies a Face-Centered Cubic Lattice and the Anions Occupy Four Positions Inside the Unit Cell
Name: __________________________ Date: _____________ 1. In an ionic compound of formula MmXn, the metal (M) occupies a face-centered cubic lattice and the anions occupy four positions inside the unit cell. Determine the formula of the compound. A) MX B) MX2 C) M2X D) MX3 E) M3X 2. Which one of the following is not a property of gases? A) particles in definite positions B) relatively low densities C) fills any container completely D) expand upon heating E) easily compressed 3. Give the systematic name for the following binary compound: S2F10 A) Disulfur decafluoride B) Sulfur fluoride C) Sulfur decafluoride D) Disulfur pentafluoride E) Disulfur fluoride 4. Which one of the following is not one of the hypotheses of Dalton's atomic theory? A) Atoms are composed of protons, neutrons, and electrons. B) All atoms of a given element are identical; the atoms of different elements are different and have different properties. C) Atoms of an element are not changed into different types of atoms by chemical reactions: atoms are neither created nor destroyed in chemical reactions. D) Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms. E) Each element is composed of extremely small particles called atoms. Page 1 5. What is the molar mass (g/mol) of sodium sulfite? A) 126.05 B) 142.05 C) 119.05 D) 103.05 E) 149.03 6. Ammonia reacts with oxygen gas to form nitric oxide (NO) and water vapour as follows: 4NH3 + 5O2 → 4NO + 6H2O What is the maximum amount of water that may be produced if 40.0 g NH3 and 50.0 g O2 are mixed and allowed to react? A) 1.88 mol B) 1.56 mol C) 3.52 mol D) 3.91 mol E) 2.35 mol 7.