Molecular Interactions Among the Intermediate Filament Proteins Paranemin, Synemin and Desmin and Their Localization During Skeletal Muscle Cell Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ITRAQ-Based Quantitative Proteomic Analysis of Processed Euphorbia Lathyris L
Zhang et al. Proteome Science (2018) 16:8 https://doi.org/10.1186/s12953-018-0136-6 RESEARCH Open Access ITRAQ-based quantitative proteomic analysis of processed Euphorbia lathyris L. for reducing the intestinal toxicity Yu Zhang1, Yingzi Wang1*, Shaojing Li2*, Xiuting Zhang1, Wenhua Li1, Shengxiu Luo1, Zhenyang Sun1 and Ruijie Nie1 Abstract Background: Euphorbia lathyris L., a Traditional Chinese medicine (TCM), is commonly used for the treatment of hydropsy, ascites, constipation, amenorrhea, and scabies. Semen Euphorbiae Pulveratum, which is another type of Euphorbia lathyris that is commonly used in TCM practice and is obtained by removing the oil from the seed that is called paozhi, has been known to ease diarrhea. Whereas, the mechanisms of reducing intestinal toxicity have not been clearly investigated yet. Methods: In this study, the isobaric tags for relative and absolute quantitation (iTRAQ) in combination with the liquid chromatography-tandem mass spectrometry (LC-MS/MS) proteomic method was applied to investigate the effects of Euphorbia lathyris L. on the protein expression involved in intestinal metabolism, in order to illustrate the potential attenuated mechanism of Euphorbia lathyris L. processing. Differentially expressed proteins (DEPs) in the intestine after treated with Semen Euphorbiae (SE), Semen Euphorbiae Pulveratum (SEP) and Euphorbiae Factor 1 (EFL1) were identified. The bioinformatics analysis including GO analysis, pathway analysis, and network analysis were done to analyze the key metabolic pathways underlying the attenuation mechanism through protein network in diarrhea. Western blot were performed to validate selected protein and the related pathways. Results: A number of differentially expressed proteins that may be associated with intestinal inflammation were identified. -

Keratins Couple with the Nuclear Lamina and Regulate Proliferation in Colonic Epithelial Cells Carl-Gustaf A
bioRxiv preprint doi: https://doi.org/10.1101/2020.06.22.164467; this version posted June 22, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Keratins couple with the nuclear lamina and regulate proliferation in colonic epithelial cells Carl-Gustaf A. Stenvall1*, Joel H. Nyström1*, Ciarán Butler-Hallissey1,5, Stephen A. Adam2, Roland Foisner3, Karen M. Ridge2, Robert D. Goldman2, Diana M. Toivola1,4 1 Cell Biology, Biosciences, Faculty of Science and Engineering, Åbo Akademi University, Turku, Finland 2 Department of Cell and Developmental Biology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA 3 Max Perutz Labs, Medical University of Vienna, Vienna Biocenter Campus (VBC), Vienna, Austria 4 Turku Center for Disease Modeling, Turku, Finland 5 Turku Bioscience Centre, University of Turku and Åbo Akademi University, Turku, Finland * indicates equal contribution Running Head: Colonocyte keratins couple to nuclear lamina Corresponding author: Diana M. Toivola Cell Biology/Biosciences, Faculty of Science and Engineering, Åbo Akademi University Tykistökatu 6A, FIN-20520 Turku, Finland Telephone: +358 2 2154092 E-mail: [email protected] Keywords: Keratins, lamin, intermediate filament, colon epithelial cells, LINC proteins, proliferation, pRb, YAP bioRxiv preprint doi: https://doi.org/10.1101/2020.06.22.164467; this version posted June 22, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Regulation of Titin-Based Cardiac Stiffness by Unfolded Domain Oxidation (Undox)
Regulation of titin-based cardiac stiffness by unfolded domain oxidation (UnDOx) Christine M. Loeschera,1, Martin Breitkreuzb,1, Yong Lia, Alexander Nickelc, Andreas Ungera, Alexander Dietld, Andreas Schmidte, Belal A. Mohamedf, Sebastian Kötterg, Joachim P. Schmitth, Marcus Krügere,i, Martina Krügerg, Karl Toischerf, Christoph Maackc, Lars I. Leichertj, Nazha Hamdanib, and Wolfgang A. Linkea,2 aInstitute of Physiology II, University of Munster, 48149 Munster, Germany; bInstitute of Physiology, Ruhr University Bochum, 44801 Bochum, Germany; cComprehensive Heart Failure Center Wuerzburg, University Clinic Wuerzburg, 97078 Wuerzburg, Germany; dDepartment of Internal Medicine II, University Hospital Regensburg, 93053 Regensburg, Germany; eInstitute for Genetics, University of Cologne, 50931 Cologne, Germany; fDepartment of Cardiology and Pneumology, University Medicine Goettingen, 37075 Goettingen, Germany; gDepartment of Cardiovascular Physiology, Heinrich Heine University, 40225 Düsseldorf, Germany; hDepartment of Pharmacology and Clinical Pharmacology, Heinrich Heine University, 40225 Düsseldorf, Germany; iCenter for Molecular Medicine and Excellence Cluster "Cellular Stress Responses in Aging-Associated Diseases" (CECAD), University of Cologne, 50931 Cologne, Germany; and jInstitute for Biochemistry and Pathobiochemistry, Ruhr University Bochum, 44801 Bochum, Germany Edited by Jonathan Seidman, Harvard University, Boston, MA, and approved August 12, 2020 (received for review March 14, 2020) The relationship between oxidative stress and -

Proteomics in Forensic Analysis: Applications for Human Samples
applied sciences Review Proteomics in Forensic Analysis: Applications for Human Samples Van-An Duong 1,† , Jong-Moon Park 1,† , Hee-Joung Lim 2,* and Hookeun Lee 1,* 1 College of Pharmacy, Gachon University, Incheon 21936, Korea; [email protected] (V.-A.D.); [email protected] (J.-M.P.) 2 Forensic Science Center for Odor Fingerprint Analysis, Police Science Institute, Korean National Police University, Asan 31539, Korea * Correspondence: [email protected] (H.-J.L.); [email protected] (H.L.); Tel.: +82-41-968-2893 (H.-J.L.); +82-32-820-4927 (H.L.) † These authors contributed equally. Abstract: Proteomics, the large-scale study of all proteins of an organism or system, is a powerful tool for studying biological systems. It can provide a holistic view of the physiological and biochemical states of given samples through identification and quantification of large numbers of peptides and proteins. In forensic science, proteomics can be used as a confirmatory and orthogonal technique for well-built genomic analyses. Proteomics is highly valuable in cases where nucleic acids are absent or degraded, such as hair and bone samples. It can be used to identify body fluids, ethnic group, gender, individual, and estimate post-mortem interval using bone, muscle, and decomposition fluid samples. Compared to genomic analysis, proteomics can provide a better global picture of a sample. It has been used in forensic science for a wide range of sample types and applications. In this review, we briefly introduce proteomic methods, including sample preparation techniques, data acquisition using liquid chromatography-tandem mass spectrometry, and data analysis using database search, Citation: Duong, V.-A.; Park, J.-M.; spectral library search, and de novo sequencing. -

Postmortem Changes in the Myofibrillar and Other Cytoskeletal Proteins in Muscle
BIOCHEMISTRY - IMPACT ON MEAT TENDERNESS Postmortem Changes in the Myofibrillar and Other C'oskeletal Proteins in Muscle RICHARD M. ROBSON*, ELISABETH HUFF-LONERGAN', FREDERICK C. PARRISH, JR., CHIUNG-YING HO, MARVIN H. STROMER, TED W. HUIATT, ROBERT M. BELLIN and SUZANNE W. SERNETT introduction filaments (titin), and integral Z-line region (a-actinin, Cap Z), as well as proteins of the intermediate filaments (desmin, The cytoskeleton of "typical" vertebrate cells contains paranemin, and synemin), Z-line periphery (filamin) and three protein filament systems, namely the -7-nm diameter costameres underlying the cell membrane (filamin, actin-containing microfilaments, the -1 0-nm diameter in- dystrophin, talin, and vinculin) are listed along with an esti- termediate filaments (IFs), and the -23-nm diameter tubu- mate of their abundance, approximate molecular weights, lin-containing microtubules (Robson, 1989, 1995; Robson and number of subunits per molecule. Because the myofibrils et al., 1991 ).The contractile myofibrils, which are by far the are the overwhelming components of the skeletal muscle cell major components of developed skeletal muscle cells and cytoskeleton, the approximate percentages of the cytoskel- are responsible for most of the desirable qualities of muscle eton listed for the myofibrillar proteins (e.g., myosin, actin, foods (Robson et al., 1981,1984, 1991 1, can be considered tropomyosin, a-actinin, etc.) also would represent their ap- the highly expanded corollary of the microfilament system proximate percentages of total myofibrillar protein. of non-muscle cells. The myofibrils, IFs, cell membrane skel- eton (complex protein-lattice subjacent to the sarcolemma), Some Important Characteristics, Possible and attachment sites connecting these elements will be con- Roles, and Postmortem Changes of Key sidered as comprising the muscle cell cytoskeleton in this Cytoskeletal Proteins review. -

Α-Actinin/Titin Interaction: a Dynamic and Mechanically Stable Cluster of Bonds in the Muscle Z-Disk
α-Actinin/titin interaction: A dynamic and mechanically stable cluster of bonds in the muscle Z-disk Marco Grisona, Ulrich Merkela, Julius Kostanb, Kristina Djinovic-Carugob,c, and Matthias Riefa,d,1 aPhysik Department E22, Technische Universität München, 85748 Garching, Germany; bDepartment of Structural and Computational Biology, Max F. Perutz Laboratories, University of Vienna, A-1030 Vienna, Austria; cDepartment of Biochemistry, Faculty of Chemistry and Chemical Technology, University of Ljubljana, SI-1000 Ljubljana, Slovenia; and dMunich Center for Integrated Protein Science, 81377 Munich, Germany Edited by James A. Spudich, Stanford University School of Medicine, Stanford, CA, and approved December 16, 2016 (received for review August 2, 2016) Stable anchoring of titin within the muscle Z-disk is essential for In humans, the isoforms of titin exhibit four to seven Z-repeats preserving muscle integrity during passive stretching. One of the (15, 16, 20). The structure of the EF3-4 hands complex with titin main candidates for anchoring titin in the Z-disk is the actin cross- Z-repeat 7 shows the bound Z-repeat in an α-helical confor- linker α-actinin. The calmodulin-like domain of α-actinin binds to mation (21). In solution assays, binding affinities of various the Z-repeats of titin. However, the mechanical and kinetic prop- Z-repeats to EF3-4 were determined to lie in the micromolar erties of this important interaction are still unknown. Here, we use range (22). Micromolar affinity points only to a moderately a dual-beam optical tweezers assay to study the mechanics of this stable interaction, the kinetics of which are unknown. -

Keratin 1 Maintains Skin Integrity and Participates in an Inflammatory
Research Article 5269 Keratin 1 maintains skin integrity and participates in an inflammatory network in skin through interleukin-18 Wera Roth1, Vinod Kumar1, Hans-Dietmar Beer2, Miriam Richter1, Claudia Wohlenberg3, Ursula Reuter3, So¨ ren Thiering1, Andrea Staratschek-Jox4, Andrea Hofmann4, Fatima Kreusch4, Joachim L. Schultze4, Thomas Vogl5, Johannes Roth5, Julia Reichelt6, Ingrid Hausser7 and Thomas M. Magin1,* 1Translational Centre for Regenerative Medicine (TRM) and Institute of Biology, University of Leipzig, 04103 Leipzig, Germany 2University Hospital, Department of Dermatology, University of Zurich, 8006 Zurich, Switzerland 3Institute of Biochemistry and Molecular Biology, Division of Cell Biochemistry, University of Bonn, 53115 Bonn, Germany 4Department of Genomics and Immunoregulation, LIMES Institute, University of Bonn, 53115 Bonn, Germany 5Institute of Immunology, University of Mu¨nster, 48149 Mu¨nster, Germany 6Institute of Cellular Medicine and North East England Stem Cell Institute, Newcastle University, Newcastle upon Tyne NE2 4HH, UK 7Universita¨ts-Hautklinik, Ruprecht-Karls-Universita¨t Heidelberg, 69120 Heidelberg, Germany *Author for correspondence ([email protected]) Accepted 8 October 2012 Journal of Cell Science 125, 5269–5279 ß 2012. Published by The Company of Biologists Ltd doi: 10.1242/jcs.116574 Summary Keratin 1 (KRT1) and its heterodimer partner keratin 10 (KRT10) are major constituents of the intermediate filament cytoskeleton in suprabasal epidermis. KRT1 mutations cause epidermolytic ichthyosis in humans, characterized by loss of barrier integrity and recurrent erythema. In search of the largely unknown pathomechanisms and the role of keratins in barrier formation and inflammation control, we show here that Krt1 is crucial for maintenance of skin integrity and participates in an inflammatory network in murine keratinocytes. -
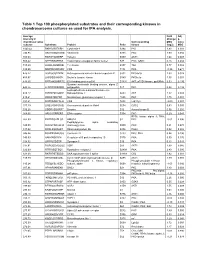
Table 1 Top 100 Phosphorylated Substrates and Their Corresponding Kinases in Chondrosarcoma Cultures As Used for IPA Analysis
Table 1 Top 100 phosphorylated substrates and their corresponding kinases in chondrosarcoma cultures as used for IPA analysis. Average Fold Adj intensity in Change p- chondrosarcoma Corresponding MSC value cultures Substrate Protein Psite kinase (log2) MSC 1043.42 RKKKVSSTKRH Cytohesin-1 S394 PKC 1.83 0.001 746.95 RKGYRSQRGHS Vitronectin S381 PKC 1.00 0.056 709.03 RARSTSLNERP Tuberin S939 AKT1 1.64 0.008 559.42 SPPRSSLRRSS Transcription elongation factor A-like1 S37 PKC; GSK3 0.18 0.684 515.29 LRRSLSRSMSQ Telethonin S157 Titin 0.77 0.082 510.00 MQPDNSSDSDY CD5 T434 PKA -0.35 0.671 476.27 GGRGGSRARNL Heterogeneous nuclear ribonucleoprotein K S302 PKCdelta 1.03 0.028 455.97 LKPGSSHRKTK Bruton's tyrosine kinase S180 PKCbeta 1.55 0.001 444.65 RRRMASMQRTG E1A binding protein p300 S1834 AKT; p70S6 kinase; pp90Rsk 0.53 0.195 Guanine nucleotide binding protein, alpha Z 440.26 HLRSESQRQRR polypeptide S27 PKC 0.88 0.199 6-phosphofructo-2-kinase/fructose-2,6- 424.12 RPRNYSVGSRP biphosphatase 2 S483 AKT 1.32 0.003 419.61 KKKIATRKPRF Metabotropic glutamate receptor 1 T695 PKC 1.75 0.001 391.21 DNSSDSDYDLH CD5 T453 Lck; Fyn -2.09 0.001 377.39 LRQLRSPRRAQ Ras associated protein Rab4 S204 CDC2 0.63 0.091 376.28 SSQRVSSYRRT Desmin S12 Aurora kinase B 0.56 0.255 369.05 ARIGGSRRERS EP4 receptor S354 PKC 0.29 0.543 RPS6 kinase alpha 3; PKA; 367.99 EPKRRSARLSA HMG14 S7 PKC -0.01 0.996 Peptidylglycine alpha amidating 349.08 SRKGYSRKGFD monooxygenase S930 PKC 0.21 0.678 347.92 RRRLSSLRAST Ribosomal protein S6 S236 PAK2 0.02 0.985 346.84 RSNPPSRKGSG Connexin -

Impact of Keratin Network Regulation on Migrating Cells
Impact of Keratin Network Regulation on Migrating Cells Von der Fakultät für Mathematik, Informatik und Naturwissenschaften der RWTH Aachen University zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigte Dissertation vorgelegt von Anne Pora, Ingénieur, Master aus Rueil-Malmaison, Frankreich Berichter: Univ.-Prof. Dr. Björn Kampa Univ.-Prof. Dr. med. Rudolf Leube Tag der mündlichen Prüfung: 02.04.19 Diese Dissertation ist auf den Internetseiten der Universitätsbibliothek verfügbar. This work was performed at the Institute for Molecular and Cellular Anatomy at University Hospital RWTH Aachen by the mentorship of Prof. Dr. med. Rudolf E. Leube. It was exclusively performed by myself, unless otherwise stated in the text. 1. Reviewer: Univ.-Prof. Dr. Björn Kampa 2. Reviewer: Univ.-Prof. Dr. med. Rudolf E. Leube Toulouse (FR), 30.11.18 2 Table of Contents Table of Contents 3 Chapter 1: Introduction 6 1. Cell migration 6 A key process in physiological and pathological conditions 6 Different kinds of migration 6 Influence of the environment 7 2. The cytoskeleton: a key player in cell migration 9 The cytoskeleton 9 Actin and focal adhesions 9 Microtubules 12 Keratin intermediate filaments and hemidesmosomes 13 Cross-talk between keratin intermediate filaments and others 22 cytoskeletal components Imaging cytoskeletal dynamics in migrating cells 24 3. Objectives 26 Chapter 2: Materials and Methods 27 1. Cell culture conditions 27 2. Keratin extraction and immunoblotting 28 3. Immunofluorescence 32 4. Plasmid constructs and DNA transfection into cultured cells 34 5. Drug treatments 35 6. Micropatterning 35 7. Preparation of elastic substrates 36 8. Imaging conditions 36 3 9. -

Functional Analysis of Tropomyosin Isoforms in Vivo
FUNCTIONAL ANALYSIS OF TROPOMYOSIN ISOFORMS IN VIVO by Aeri Cho A dissertation submitted to Johns Hopkins University in conformity with the requirements for the degree of Doctor of Philosophy Baltimore, Maryland March, 2015 Abstract Precise regulation of a dynamic actin cytoskeleton is an essential function of animal cells without which vesicle trafficking, cytokinesis, cell adhesion and cell movement would be impossible. Therefore, understanding the mechanisms underlying actin dynamics is fundamental for understanding basic cellular biology as well as gaining insight into mechanisms of embryonic development, tissue homeostasis and regeneration, and pathological processes such as inflammation and tumor metastasis. Actin filaments are a major source of the protrusive and contractile forces that drive many cellular behaviors. Contractile forces require the action of non-muscle myosin II, which assembles onto actin filaments to form acto-myosin. The interaction between actin and myosin can occur spontaneously in vitro, but in cells it is regulated by accessory proteins including Tropomyosins (Tms). A complete understanding of Tm function has been elusive due in part to the large number of isoforms: 44 predicted isoforms from 4 genes in humans. The goal of this study was to decipher the functional roles of different Tm isoforms at the molecular, cellular and tissue levels in vivo. Drosophila egg chambers are a genetically tractable system that expresses far fewer Tm isoforms than mammalian cells. We identified three tropomyosin isoforms expressed in follicle cells, including one previously annotated as muscle-specific. We generated and characterized isoform- specific antibodies, RNAi lines, and mutant alleles, and discovered that they function non-redundantly in the two cell types we studied: border cells, a well-studied example of collective migration, and epithelial follicle cells, which develop contractile stress fibers that shape the egg chamber. -

Association of Titin and Myosin Heavy Chain in Developing Skeletal Muscle (Myogenesis/Cytoskeleton/Assembly in Vvo) W
Proc. Natl. Acad. Sci. USA Vol. 89, pp. 74%-7500, August 1992 Cell Biology Association of titin and myosin heavy chain in developing skeletal muscle (myogenesis/cytoskeleton/assembly in vvo) W. B. ISAACS*, I. S. KIM, A. STRUVE, AND A. B. FULTONt Department of Biochemistry, University of Iowa, Iowa City, IA 52242 Communicated by Sheldon Penman, May 22, 1992 ABSTRACT To understand molecular interactions that deficient medium (10). After labeling, cultures either were organize developing myoflbrils, we examined the biosynthesis extracted immediately or were chased by adding complete and interaction of titin and myosin heavy chain in cultures of medium supplemented with 2 mM unlabeled methionine and developing muscle. Use of pulse-labeling, immunoprecipita- incubating at 370C for various times before extraction. Ex- tion, and a reversible cross-linking procedure demonstrates tractions used 0.5% Triton X-100 in extraction buffer (100 that within minutes of synthesis, titin and myosin heavy chain mM KCI/10 mM Pipes, pH 6.8/300 mM sucrose/2 mM can be chemically cross-linked into very large, detergent- MgCI2/1 mM EGTA) containing protease inhibitors (1 mM resistant complexes retaining many features of intact myo- phenylmethylsulfonyl fluoride and 100 mM leupeptin; ref. tubes. These complexes, predominantly of titin and myosin, 11). occur very early in myofibrillogenesis as well as later. These Immunoprecipitation. Titin was precipitated by using a data suggest that synthesis and assembly oftitin and myosin are mouse monoclonal antibody (mAb), AMF-1, as described temporally and spatially coordinated in nascent myofibrils and (10). Muscle-specific myosin heavy chain (hereafter myosin) support the hypothesis that titin molecules help to organize was precipitated with mAb MF-20 (12), a gift ofD. -

Titin N2A Domain and Its Interactions at the Sarcomere
International Journal of Molecular Sciences Review Titin N2A Domain and Its Interactions at the Sarcomere Adeleye O. Adewale and Young-Hoon Ahn * Department of Chemistry, Wayne State University, Detroit, MI 48202, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-(313)-577-1384 Abstract: Titin is a giant protein in the sarcomere that plays an essential role in muscle contraction with actin and myosin filaments. However, its utility goes beyond mechanical functions, extending to versatile and complex roles in sarcomere organization and maintenance, passive force, mechanosens- ing, and signaling. Titin’s multiple functions are in part attributed to its large size and modular structures that interact with a myriad of protein partners. Among titin’s domains, the N2A element is one of titin’s unique segments that contributes to titin’s functions in compliance, contraction, structural stability, and signaling via protein–protein interactions with actin filament, chaperones, stress-sensing proteins, and proteases. Considering the significance of N2A, this review highlights structural conformations of N2A, its predisposition for protein–protein interactions, and its multiple interacting protein partners that allow the modulation of titin’s biological effects. Lastly, the nature of N2A for interactions with chaperones and proteases is included, presenting it as an important node that impacts titin’s structural and functional integrity. Keywords: titin; N2A domain; protein–protein interaction 1. Introduction Citation: Adewale, A.O.; Ahn, Y.-H. The complexity of striated muscle is defined by the intricate organization of its com- Titin N2A Domain and Its ponents [1]. The involuntary cardiac and voluntary skeletal muscles are the primary types Interactions at the Sarcomere.