Biological Functions of Cytokeratin 18 in Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In Situlocalization of Cytoskeletal Elements in the Human Trabecular
Investigative Ophthalmology & Visual Science. Vol. 31. No. 9. September 1990 Cops right £• Association lor Research in Vision and Ophthalmology In Situ Localization of Cytoskeletal Elements in the Human Trabecular Meshwork and Cornea Robert N. Weinreb* and Mark I. Ryderf The authors compared cytoskeletal elements of the in situ human trabccular-mcshwork cell with in situ human corneal cells using indirect immunofluorcsccncc staining for tubulin and intermediate filaments (vimentin, cytokeratin, and desmin) and NBD-phallacidin staining for f-actin using both fixed frozen and unfixed frozen sections from postmortem eyes. Both f-actin and tubulin were found throughout the cell body of trabecular-meshwork cells, keratocytes, corneal endothelium, and corneal epithelium. The f-actin staining pattern was concentrated at the cell periphery of these four cell types. Vimentin stain was intensely localized in focal areas of the trabecular-meshwork cell, keratocytes, and throughout the corneal cndothelium. A general anticytokeratin antibody was intensely localized in corneal epithelium and endothelium. However, PKK-1 anticytokeratin antibody was seen only in superficial layers of corneal epithelium and not in corneal endothelium. The 4.62 anticytokeratin antibody was not observed in either corneal epithelium or endothelium. None of these three cytokera- tin antibodies were seen in trabccular-mcshwork cells or keratocytes. Desmin stain was not noted in any of these cell types. In general, cytoskeletal staining of unfixed frozen sections showed a similar staining pattern for f-actin and tubulin but a more uniform and intense staining pattern for vimentin and cytokcratin compared with fixed frozen material. The authors conclude that these cytoskclctal stains can differentiate human Irabeciilar-meshwork cells from cells of the cornea in situ. -

Decreased Expression of Profilin 2 in Oral Squamous Cell Carcinoma and Its Clinicopathological Implications
ONCOLOGY REPORTS 26: 813-823, 2011 Decreased expression of profilin 2 in oral squamous cell carcinoma and its clinicopathological implications C.Y. MA1,2, C.P. ZHANG1,2, L.P. ZHONG1,2, H.Y. PAN1,2, W.T. CHEN1,2, L.Z. WANG3, O.W. ANDREW4, T. JI1 and W. HAN1,2 1Department of Oral and Maxillofacial Surgery, Ninth People's Hospital, College of Stomatology; 2Shanghai Key Laboratory of Stomatology and Shanghai Research Institute of Stomatology; 3Department of Oral Pathology, Ninth People's Hospital, College of Stomatology, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, P.R. China; 4Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, National University of Singapore, Singapore 119074, Singapore Received February 8, 2011; Accepted April 11, 2011 DOI: 10.3892/or.2011.1365 Abstract. Profilins are small proteins essential for many clinical and pathological significance. In conclusion, PFN2 normal cellular dynamics and constitute one of the crucial can be utilized as both a potential suppressor marker and a components of actin-based cellular motility. Several recent prognostic protein for OSCC. The function of PFN2 may be to studies have implicated a role for the profilin (PFN) family in regulate the N-WASP/Arp2/3 signaling pathway. cancer pathogenesis and progression. However, their expression and promising functions are largely unknown in oral squamous Introduction cell carcinoma (OSCC). In this study, we analyzed the correlation between PFN1 and PFN2 expression in vitro and Oral squamous cell carcinoma (OSCC) is a significant public in vivo. The protein expression levels were roughly compared health problem with >300,000 new cases being diagnosed between cell lines (HIOEC, HB96) with the employment of annually worldwide (1). -

A Significant Soluble Keratin Fraction In
Journal of Cell Science 105, 433-444 (1993) 433 Printed in Great Britain © The Company of Biologists Limited 1993 A significant soluble keratin fraction in ‘simple’ epithelial cells Lack of an apparent phosphorylation and glycosylation role in keratin solubility Chih-Fong Chou*, Carrie L. Riopel, Lusijah S. Rott and M. Bishr Omary† Palo Alto Veterans Administration Medical Center and the Digestive Disease Center at Stanford University, School of Medicine, 3801 Miranda Avenue, GI 111, Palo Alto, CA 94304, USA *Author for reprint requests †Author for correspondence SUMMARY We studied the solubility of keratin polypeptides 8 and aments in vitro as determined by electron microscopy. 18 (K8/18), which are the predominant intermediate fil- Cross-linking of soluble K8/18 followed by immunopre- aments in the human colonic epithelial cell line HT29. cipitation resulted in dimeric and tetrameric forms, We find that asynchronously growing cells (G0/G1 stage based on migration in SDS-polyacrylamide gels. In of the cell cycle) have a substantial pool of soluble ker- addition, cross-linked and native soluble K8/18 showed atin that constitutes approx. 5% of total cellular ker- similar migration on nondenaturing gels and similar atin. This soluble keratin pool was observed after sedimentation after sucrose density gradient centrifu- immunoprecipitation of K8/18 from the cytosolic frac- gation. Our results indicate that simple epithelial ker- tion of cells disrupted using three detergent-free meth- atins are appreciably more soluble than previously rec- ods. Several other cell lines showed a similar significant ognized. The soluble keratin form is assembly competent soluble cytosolic K8/18 pool. -

Genetic Background Effects of Keratin 8 and 18 in a DDC-Induced Hepatotoxicity and Mallory-Denk Body Formation Mouse Model
Laboratory Investigation (2012) 92, 857–867 & 2012 USCAP, Inc All rights reserved 0023-6837/12 $32.00 Genetic background effects of keratin 8 and 18 in a DDC-induced hepatotoxicity and Mallory-Denk body formation mouse model Johannes Haybaeck1, Cornelia Stumptner1, Andrea Thueringer1, Thomas Kolbe2, Thomas M Magin3, Michael Hesse4, Peter Fickert5, Oleksiy Tsybrovskyy1, Heimo Mu¨ller1, Michael Trauner5,6, Kurt Zatloukal1 and Helmut Denk1 Keratin 8 (K8) and keratin 18 (K18) form the major hepatocyte cytoskeleton. We investigated the impact of genetic loss of either K8 or K18 on liver homeostasis under toxic stress with the hypothesis that K8 and K18 exert different functions. krt8À/À and krt18À/À mice crossed into the same 129-ola genetic background were treated by acute and chronic ad- ministration of 3,5-diethoxy-carbonyl-1,4-dihydrocollidine (DDC). In acutely DDC-intoxicated mice, macrovesicular steatosis was more pronounced in krt8À/À and krt18À/À compared with wild-type (wt) animals. Mallory-Denk bodies (MDBs) appeared in krt18À/À mice already at an early stage of intoxication in contrast to krt8À/À mice that did not display MDB formation when fed with DDC. Keratin-deficient mice displayed significantly lower numbers of apoptotic hepatocytes than wt animals. krt8À/À, krt18À/À and control mice displayed comparable cell proliferation rates. Chronically DDC-intoxicated krt18À/À and wt mice showed a similarly increased degree of steatohepatitis with hepatocyte ballooning and MDB formation. In krt8À/À mice, steatosis was less, ballooning, and MDBs were absent. krt18À/À mice developed MDBs whereas krt8À/À mice on the same genetic background did not, highlighting the significance of different structural properties of keratins. -
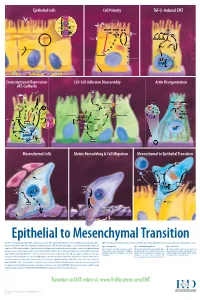
Epithelial to Mesenchymal Transition
Epithelial Cells Cell Polarity TGF-b-Induced EMT MUC-1 O-glycosylation Epithelial Cells ZO-1 Occludin Apical Membrane Tight F-Actin Microvilli Junction Claudin F-Actin p120 β-Catenin Adherens F-Actin Ezrin TGF-β dimer Junction E-Cadherin α-Catenin Plakophilin Crumbs Complex PAR Complex Desmocollin Desmoplakin Desmosome PtdIns(4,5)P2 TGF-β RII TGF-β RI CRB Cdc42Par6 Desmoglein Cytokeratin Pals1 PatJ Tight Junction Plakoglobin aPKC Par3 Domain Smad7 Extracellular PTEN JNK ERK1/2 p38 SARA Smurf1 Cortical Actin Cytoskeleton Space Par3 ZO-1 Adherens Junction PI 3-K Domain Smad-independent Signaling (–) Smad7 Translocation Smad2/3 PtdIns(3,4,5)P3 Smad4 Smad4 NEDD4 Cytokeratin Intermediate Filaments Smad2 Smad4 Smad3 LLGL Proteasome SCRIB DLG Scribble Complex Fibronectin Twist Smad2/3 Vitronectin ZEB 1/2 Microtubule Network Smad4 N-Cadherin Snail Basolateral Membrane CoA, Collagen I Slug CoR MMPs DNA-binding (+) Claudin Desmoplakin Transcription Factor Occludin Cytokeratins E-Cadherin Plakoglobin Integrins β α Nidogen-1/Entactin Perlecan Laminin Collagen IV Transcriptional Repression Cell-Cell Adhesion Disassembly Actin Reorganization of E-Cadherin TGF-β dimer EGF TGF-β RII TGF-β RI IGF FGF Receptor TNF-α Tyrosine Kinase Par6 TNF RI Apical Focal Adhesion Constriction Actin Depolymerization F-Actin Smurf1 Occludin Wnt Frizzled Myosin II Ras RhoA α-Actinin Myosin II ROCK AxinCK1 Dishevelled GSK-3 PI 3-K Src Zyxin MLC Phosphatase APC Proteasome FAK Vinculin RhoA ILK Talin (Inactive) Hakai Talin FAK F-Actin E-Cadherin LIMK Akt Paxillin FAK Stress -

ITRAQ-Based Quantitative Proteomic Analysis of Processed Euphorbia Lathyris L
Zhang et al. Proteome Science (2018) 16:8 https://doi.org/10.1186/s12953-018-0136-6 RESEARCH Open Access ITRAQ-based quantitative proteomic analysis of processed Euphorbia lathyris L. for reducing the intestinal toxicity Yu Zhang1, Yingzi Wang1*, Shaojing Li2*, Xiuting Zhang1, Wenhua Li1, Shengxiu Luo1, Zhenyang Sun1 and Ruijie Nie1 Abstract Background: Euphorbia lathyris L., a Traditional Chinese medicine (TCM), is commonly used for the treatment of hydropsy, ascites, constipation, amenorrhea, and scabies. Semen Euphorbiae Pulveratum, which is another type of Euphorbia lathyris that is commonly used in TCM practice and is obtained by removing the oil from the seed that is called paozhi, has been known to ease diarrhea. Whereas, the mechanisms of reducing intestinal toxicity have not been clearly investigated yet. Methods: In this study, the isobaric tags for relative and absolute quantitation (iTRAQ) in combination with the liquid chromatography-tandem mass spectrometry (LC-MS/MS) proteomic method was applied to investigate the effects of Euphorbia lathyris L. on the protein expression involved in intestinal metabolism, in order to illustrate the potential attenuated mechanism of Euphorbia lathyris L. processing. Differentially expressed proteins (DEPs) in the intestine after treated with Semen Euphorbiae (SE), Semen Euphorbiae Pulveratum (SEP) and Euphorbiae Factor 1 (EFL1) were identified. The bioinformatics analysis including GO analysis, pathway analysis, and network analysis were done to analyze the key metabolic pathways underlying the attenuation mechanism through protein network in diarrhea. Western blot were performed to validate selected protein and the related pathways. Results: A number of differentially expressed proteins that may be associated with intestinal inflammation were identified. -

Structures of the ß-Keratin Filaments and Keratin Intermediate Filaments in the Epidermal Appendages of Birds and Reptiles (Sauropsids)
G C A T T A C G G C A T genes Review Structures of the ß-Keratin Filaments and Keratin Intermediate Filaments in the Epidermal Appendages of Birds and Reptiles (Sauropsids) David A.D. Parry School of Fundamental Sciences, Massey University, Private Bag 11-222, Palmerston North 4442, New Zealand; [email protected]; Tel.: +64-6-9517620; Fax: +64-6-3557953 Abstract: The epidermal appendages of birds and reptiles (the sauropsids) include claws, scales, and feathers. Each has specialized physical properties that facilitate movement, thermal insulation, defence mechanisms, and/or the catching of prey. The mechanical attributes of each of these appendages originate from its fibril-matrix texture, where the two filamentous structures present, i.e., the corneous ß-proteins (CBP or ß-keratins) that form 3.4 nm diameter filaments and the α-fibrous molecules that form the 7–10 nm diameter keratin intermediate filaments (KIF), provide much of the required tensile properties. The matrix, which is composed of the terminal domains of the KIF molecules and the proteins of the epidermal differentiation complex (EDC) (and which include the terminal domains of the CBP), provides the appendages, with their ability to resist compression and torsion. Only by knowing the detailed structures of the individual components and the manner in which they interact with one another will a full understanding be gained of the physical properties of the tissues as a whole. Towards that end, newly-derived aspects of the detailed conformations of the two filamentous structures will be discussed and then placed in the context of former knowledge. -

Identification of Trichoplein, a Novel Keratin Filament- Binding Protein
Research Article 1081 Identification of trichoplein, a novel keratin filament- binding protein Miwako Nishizawa1,*, Ichiro Izawa1,*, Akihito Inoko1,*, Yuko Hayashi1, Koh-ichi Nagata1, Tomoya Yokoyama1,2, Jiro Usukura3 and Masaki Inagaki1,‡ 1Division of Biochemistry, Aichi Cancer Center Research Institute, 1-1 Kanokoden, Chikusa-ku, Nagoya 464-8681, Japan 2Department of Dermatology, Mie University Faculty of Medicine, 2-174 Edobashi, Tsu 514-8507, Japan 3Department of Anatomy and Cell Biology, Nagoya University School of Medicine, 65 Tsurumai, Showa-ku, Nagoya 466-8550, Japan *These authors contributed equally to this work ‡Author for correspondence (e-mail: [email protected]) Accepted 29 November 2004 Journal of Cell Science 118, 1081-1090 Published by The Company of Biologists 2005 doi:10.1242/jcs.01667 Summary Keratins 8 and 18 (K8/18) are major components of the antibody in a complex with K8/18 and immunostaining intermediate filaments (IFs) of simple epithelia. We report revealed that trichoplein colocalized with K8/18 filaments here the identification of a novel protein termed in HeLa cells. In polarized Caco-2 cells, trichoplein trichoplein. This protein shows a low degree of sequence colocalized not only with K8/18 filaments in the apical similarity to trichohyalin, plectin and myosin heavy chain, region but also with desmoplakin, a constituent of and is a K8/18-binding protein. Among interactions desmosomes. In the absorptive cells of the small intestine, between trichoplein and various IF proteins that we trichoplein colocalized with K8/18 filaments at the apical tested using two-hybrid methods, trichoplein interacted cortical region, and was also concentrated at desmosomes. -
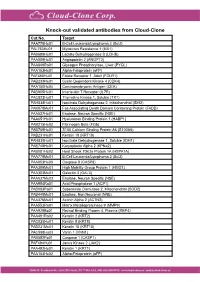
Knock-Out Validated Antibodies from Cloud-Clone Cat.No
Knock-out validated antibodies from Cloud-Clone Cat.No. Target PAA778Hu01 B-Cell Leukemia/Lymphoma 2 (Bcl2) PAL763Hu01 Myxovirus Resistance 1 (MX1) PAB698Hu01 Lactate Dehydrogenase B (LDHB) PAA009Hu01 Angiopoietin 2 (ANGPT2) PAA849Ra01 Glycogen Phosphorylase, Liver (PYGL) PAA153Hu01 Alpha-Fetoprotein (aFP) PAF460Hu01 Folate Receptor 1, Adult (FOLR1) PAB233Hu01 Cyclin Dependent Kinase 4 (CDK4) PAA150Hu04 Carcinoembryonic Antigen (CEA) PAB905Hu01 Interleukin 7 Receptor (IL7R) PAC823Hu01 Thymidine Kinase 1, Soluble (TK1) PAH838Hu01 Isocitrate Dehydrogenase 2, mitochondrial (IDH2) PAK078Mu01 Fas Associating Death Domain Containing Protein (FADD) PAA537Hu01 Enolase, Neuron Specific (NSE) PAA651Hu01 Hyaluronan Binding Protein 1 (HABP1) PAB215Hu02 Fibrinogen Beta (FGb) PAB769Hu01 S100 Calcium Binding Protein A6 (S100A6) PAB231Hu01 Keratin 18 (KRT18) PAH839Hu01 Isocitrate Dehydrogenase 1, Soluble (IDH1) PAE748Hu01 Karyopherin Alpha 2 (KPNa2) PAB081Hu02 Heat Shock 70kDa Protein 1A (HSPA1A) PAA778Mu01 B-Cell Leukemia/Lymphoma 2 (Bcl2) PAA853Hu03 Caspase 8 (CASP8) PAA399Mu01 High Mobility Group Protein 1 (HMG1) PAA303Mu01 Galectin 3 (GAL3) PAA537Mu02 Enolase, Neuron Specific (NSE) PAA994Ra01 Acid Phosphatase 1 (ACP1) PAB083Ra01 Superoxide Dismutase 2, Mitochondrial (SOD2) PAB449Mu01 Enolase, Non Neuronal (NNE) PAA376Mu01 Actinin Alpha 2 (ACTN2) PAA553Ra01 Matrix Metalloproteinase 9 (MMP9) PAA929Bo01 Retinol Binding Protein 4, Plasma (RBP4) PAA491Ra02 Keratin 2 (KRT2) PAC025Hu01 Keratin 8 (KRT8) PAB231Mu01 Keratin 18 (KRT18) PAC598Hu03 Vanin 1 (VNN1) -

Motility-Related Actinin Alpha-4 Is Associated with Advanced And
Laboratory Investigation (2008) 88, 602–614 & 2008 USCAP, Inc All rights reserved 0023-6837/08 $30.00 Motility-related actinin alpha-4 is associated with advanced and metastatic ovarian carcinoma Maria V Barbolina1, Brian P Adley2, David L Kelly3, Angela J Fought4, Denise M Scholtens4, Lonnie D Shea1 and M Sharon Stack5 Advanced and metastatic ovarian cancer is a leading cause of death from gynecologic malignancies. A more detailed understanding of the factors controlling invasion and metastasis may lead to novel anti-metastatic therapies. To model cellular interactions that occur during intraperitoneal metastasis, comparative cDNA microarray analysis and confirmatory real-time reverse transcription PCR (RT-PCR) were employed to uncover changes in gene expression that may occur in late stage ovarian cancer in response to microenvironmental cues, particularly native three-dimensional collagen I. Gene expression in human ovarian carcinoma tissues was evaluated on the RNA and protein level using real-time RT-PCR and immunohistochemistry. Cell invasion and migration were evaluated in a collagen invasion assay and a scratch wound assay. Three-dimensional collagen I culture led to differential expression of several genes. The role of actinin alpha-4 (ACTN4), a cytoskeleton-associated protein implicated in the regulation of cell motility, was examined in detail. ACTN4 RNA and protein expression were associated with advanced and metastatic human ovarian carcinoma. This report demonstrates that a cytoskeletal-associated protein ACTN4 is upregulated by three-dimensional collagen culture conditions, leading to increased invasion and motility of ovarian cancer cells. Expression of ACTN4 in human ovarian tumors was found to be associated with advanced-stage disease and peritoneal metastases. -

Keratins Couple with the Nuclear Lamina and Regulate Proliferation in Colonic Epithelial Cells Carl-Gustaf A
bioRxiv preprint doi: https://doi.org/10.1101/2020.06.22.164467; this version posted June 22, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Keratins couple with the nuclear lamina and regulate proliferation in colonic epithelial cells Carl-Gustaf A. Stenvall1*, Joel H. Nyström1*, Ciarán Butler-Hallissey1,5, Stephen A. Adam2, Roland Foisner3, Karen M. Ridge2, Robert D. Goldman2, Diana M. Toivola1,4 1 Cell Biology, Biosciences, Faculty of Science and Engineering, Åbo Akademi University, Turku, Finland 2 Department of Cell and Developmental Biology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA 3 Max Perutz Labs, Medical University of Vienna, Vienna Biocenter Campus (VBC), Vienna, Austria 4 Turku Center for Disease Modeling, Turku, Finland 5 Turku Bioscience Centre, University of Turku and Åbo Akademi University, Turku, Finland * indicates equal contribution Running Head: Colonocyte keratins couple to nuclear lamina Corresponding author: Diana M. Toivola Cell Biology/Biosciences, Faculty of Science and Engineering, Åbo Akademi University Tykistökatu 6A, FIN-20520 Turku, Finland Telephone: +358 2 2154092 E-mail: [email protected] Keywords: Keratins, lamin, intermediate filament, colon epithelial cells, LINC proteins, proliferation, pRb, YAP bioRxiv preprint doi: https://doi.org/10.1101/2020.06.22.164467; this version posted June 22, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Human Induced Pluripotent Stem Cell–Derived Podocytes Mature Into Vascularized Glomeruli Upon Experimental Transplantation
BASIC RESEARCH www.jasn.org Human Induced Pluripotent Stem Cell–Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation † Sazia Sharmin,* Atsuhiro Taguchi,* Yusuke Kaku,* Yasuhiro Yoshimura,* Tomoko Ohmori,* ‡ † ‡ Tetsushi Sakuma, Masashi Mukoyama, Takashi Yamamoto, Hidetake Kurihara,§ and | Ryuichi Nishinakamura* *Department of Kidney Development, Institute of Molecular Embryology and Genetics, and †Department of Nephrology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan; ‡Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, Hiroshima, Japan; §Division of Anatomy, Juntendo University School of Medicine, Tokyo, Japan; and |Japan Science and Technology Agency, CREST, Kumamoto, Japan ABSTRACT Glomerular podocytes express proteins, such as nephrin, that constitute the slit diaphragm, thereby contributing to the filtration process in the kidney. Glomerular development has been analyzed mainly in mice, whereas analysis of human kidney development has been minimal because of limited access to embryonic kidneys. We previously reported the induction of three-dimensional primordial glomeruli from human induced pluripotent stem (iPS) cells. Here, using transcription activator–like effector nuclease-mediated homologous recombination, we generated human iPS cell lines that express green fluorescent protein (GFP) in the NPHS1 locus, which encodes nephrin, and we show that GFP expression facilitated accurate visualization of nephrin-positive podocyte formation in