In Situlocalization of Cytoskeletal Elements in the Human Trabecular
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Decreased Expression of Profilin 2 in Oral Squamous Cell Carcinoma and Its Clinicopathological Implications
ONCOLOGY REPORTS 26: 813-823, 2011 Decreased expression of profilin 2 in oral squamous cell carcinoma and its clinicopathological implications C.Y. MA1,2, C.P. ZHANG1,2, L.P. ZHONG1,2, H.Y. PAN1,2, W.T. CHEN1,2, L.Z. WANG3, O.W. ANDREW4, T. JI1 and W. HAN1,2 1Department of Oral and Maxillofacial Surgery, Ninth People's Hospital, College of Stomatology; 2Shanghai Key Laboratory of Stomatology and Shanghai Research Institute of Stomatology; 3Department of Oral Pathology, Ninth People's Hospital, College of Stomatology, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, P.R. China; 4Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, National University of Singapore, Singapore 119074, Singapore Received February 8, 2011; Accepted April 11, 2011 DOI: 10.3892/or.2011.1365 Abstract. Profilins are small proteins essential for many clinical and pathological significance. In conclusion, PFN2 normal cellular dynamics and constitute one of the crucial can be utilized as both a potential suppressor marker and a components of actin-based cellular motility. Several recent prognostic protein for OSCC. The function of PFN2 may be to studies have implicated a role for the profilin (PFN) family in regulate the N-WASP/Arp2/3 signaling pathway. cancer pathogenesis and progression. However, their expression and promising functions are largely unknown in oral squamous Introduction cell carcinoma (OSCC). In this study, we analyzed the correlation between PFN1 and PFN2 expression in vitro and Oral squamous cell carcinoma (OSCC) is a significant public in vivo. The protein expression levels were roughly compared health problem with >300,000 new cases being diagnosed between cell lines (HIOEC, HB96) with the employment of annually worldwide (1). -
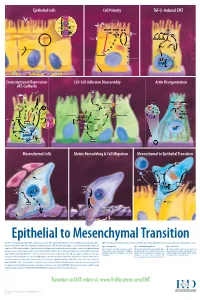
Epithelial to Mesenchymal Transition
Epithelial Cells Cell Polarity TGF-b-Induced EMT MUC-1 O-glycosylation Epithelial Cells ZO-1 Occludin Apical Membrane Tight F-Actin Microvilli Junction Claudin F-Actin p120 β-Catenin Adherens F-Actin Ezrin TGF-β dimer Junction E-Cadherin α-Catenin Plakophilin Crumbs Complex PAR Complex Desmocollin Desmoplakin Desmosome PtdIns(4,5)P2 TGF-β RII TGF-β RI CRB Cdc42Par6 Desmoglein Cytokeratin Pals1 PatJ Tight Junction Plakoglobin aPKC Par3 Domain Smad7 Extracellular PTEN JNK ERK1/2 p38 SARA Smurf1 Cortical Actin Cytoskeleton Space Par3 ZO-1 Adherens Junction PI 3-K Domain Smad-independent Signaling (–) Smad7 Translocation Smad2/3 PtdIns(3,4,5)P3 Smad4 Smad4 NEDD4 Cytokeratin Intermediate Filaments Smad2 Smad4 Smad3 LLGL Proteasome SCRIB DLG Scribble Complex Fibronectin Twist Smad2/3 Vitronectin ZEB 1/2 Microtubule Network Smad4 N-Cadherin Snail Basolateral Membrane CoA, Collagen I Slug CoR MMPs DNA-binding (+) Claudin Desmoplakin Transcription Factor Occludin Cytokeratins E-Cadherin Plakoglobin Integrins β α Nidogen-1/Entactin Perlecan Laminin Collagen IV Transcriptional Repression Cell-Cell Adhesion Disassembly Actin Reorganization of E-Cadherin TGF-β dimer EGF TGF-β RII TGF-β RI IGF FGF Receptor TNF-α Tyrosine Kinase Par6 TNF RI Apical Focal Adhesion Constriction Actin Depolymerization F-Actin Smurf1 Occludin Wnt Frizzled Myosin II Ras RhoA α-Actinin Myosin II ROCK AxinCK1 Dishevelled GSK-3 PI 3-K Src Zyxin MLC Phosphatase APC Proteasome FAK Vinculin RhoA ILK Talin (Inactive) Hakai Talin FAK F-Actin E-Cadherin LIMK Akt Paxillin FAK Stress -

Damage of Hair Follicle Stem Cells and Alteration of Keratin Expression in External Radiation-Induced Acute Alopecia
INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 30: 579-584, 2012 Damage of hair follicle stem cells and alteration of keratin expression in external radiation-induced acute alopecia NAOKI NANASHIMA, KOICHI ITO, TAKASHI ISHIKAWA, MANABU NAKANO and TOSHIYA NAKAMURA Department of Biomedical Sciences, Division of Medical Life Sciences, Hirosaki University Graduate School of Health Sciences, Hirosaki, Japan Received April 4, 2012; Accepted May 28, 2012 DOI: 10.3892/ijmm.2012.1018 Abstract. Alopecia is known as a symptom of acute radia- disturbances and blood and bone marrow disorders are known tion, yet little is known concerning the mechanism of this to occur within several hours to several weeks after 1-6 Gy of phenomenon and the alteration of hair protein profiles. To radiation exposure (4,6). examine this, 6-week-old male C57/BL6 mice were exposed Hair loss is also an effect of ARS, but little is known to 6 Gy of X-ray irradiation, which caused acute alopecia. about the mechanism underlying radiation-induced hair loss. Their hair and skin were collected, and hair proteins were In humans, hair loss is caused by radiation of more than analyzed with liquid chromatography/electrospray-ionization 3 Gy, and almost complete hair loss occurs within weeks of mass spectrometry and immunohistochemistry. No change exposure to 6 Gy (4,6). Since blood stem cells are sensitive to was observed in the composition of major hair keratins, such radiation (7), hair loss is thought to be caused by irradiation- as Krt81, Krt83 and Krt86. However, cytokeratin Krt15 and induced stem cell damage, yet no studies have investigated CD34, which are known as hair follicle stem cell markers, this hypothesis. -

Cytoskeletal Remodeling in Cancer
biology Review Cytoskeletal Remodeling in Cancer Jaya Aseervatham Department of Ophthalmology, University of Texas Health Science Center at Houston, Houston, TX 77054, USA; [email protected]; Tel.: +146-9767-0166 Received: 15 October 2020; Accepted: 4 November 2020; Published: 7 November 2020 Simple Summary: Cell migration is an essential process from embryogenesis to cell death. This is tightly regulated by numerous proteins that help in proper functioning of the cell. In diseases like cancer, this process is deregulated and helps in the dissemination of tumor cells from the primary site to secondary sites initiating the process of metastasis. For metastasis to be efficient, cytoskeletal components like actin, myosin, and intermediate filaments and their associated proteins should co-ordinate in an orderly fashion leading to the formation of many cellular protrusions-like lamellipodia and filopodia and invadopodia. Knowledge of this process is the key to control metastasis of cancer cells that leads to death in 90% of the patients. The focus of this review is giving an overall understanding of these process, concentrating on the changes in protein association and regulation and how the tumor cells use it to their advantage. Since the expression of cytoskeletal proteins can be directly related to the degree of malignancy, knowledge about these proteins will provide powerful tools to improve both cancer prognosis and treatment. Abstract: Successful metastasis depends on cell invasion, migration, host immune escape, extravasation, and angiogenesis. The process of cell invasion and migration relies on the dynamic changes taking place in the cytoskeletal components; actin, tubulin and intermediate filaments. This is possible due to the plasticity of the cytoskeleton and coordinated action of all the three, is crucial for the process of metastasis from the primary site. -

Biological Functions of Cytokeratin 18 in Cancer
Published OnlineFirst March 27, 2012; DOI: 10.1158/1541-7786.MCR-11-0222 Molecular Cancer Review Research Biological Functions of Cytokeratin 18 in Cancer Yu-Rong Weng1,2, Yun Cui1,2, and Jing-Yuan Fang1,2,3 Abstract The structural proteins cytokeratin 18 (CK18) and its coexpressed complementary partner CK8 are expressed in a variety of adult epithelial organs and may play a role in carcinogenesis. In this study, we focused on the biological functions of CK18, which is thought to modulate intracellular signaling and operates in conjunction with various related proteins. CK18 may affect carcinogenesis through several signaling pathways, including the phosphoinosi- tide 3-kinase (PI3K)/Akt, Wnt, and extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) signaling pathways. CK18 acts as an identical target of Akt in the PI3K/Akt pathway and of ERK1/2 in the ERK MAPK pathway, and regulation of CK18 by Wnt is involved in Akt activation. Finally, we discuss the importance of gaining a more complete understanding of the expression of CK18 during carcinogenesis, and suggest potential clinical applications of that understanding. Mol Cancer Res; 10(4); 1–9. Ó2012 AACR. Introduction epithelial organs, such as the liver, lung, kidney, pancreas, The intermediate filaments consist of a large number of gastrointestinal tract, and mammary gland, and are also nuclear and cytoplasmic proteins that are expressed in a expressed by cancers that arise from these tissues (7). In the tissue- and differentiation-dependent manner. The compo- absence of CK8, the CK18 protein is degraded and keratin fi intermediate filaments are not formed (8). -

Cutaneous Dermoid Cyst: Cytokeratin and Filaggrin Expression Suggesting Differentiation Towards Follicular Infundibulum and Mature Sebaceous Gland
295-299 3/7/06 18:45 Page 295 ONCOLOGY REPORTS 16: 295-299, 2006 295 Cutaneous dermoid cyst: Cytokeratin and filaggrin expression suggesting differentiation towards follicular infundibulum and mature sebaceous gland ICHIRO KUROKAWA1, KEISUKE NISHIMURA1, ARATA HAKAMADA1, KEN-ICHI ISODA1, KEI-ICHI YAMANAKA1, HITOSHI MIZUTANI1 and AIRO TSUBURA2 1Department of Dermatology, Mie University Graduate School of Medicine, Tsu, Mie 514-8507; 2Department of Pathology, Kansai Medical University, Moriguchi, Osaka 570-8506, Japan Received February 20, 2006; Accepted April 10, 2006 Abstract. We experienced two cases of cutaneous dermoid To elucidate the histogenesis of DC, we studied CK and cysts (DC). To elucidate the histogenesis of DC, we have filaggrin expression in DC using ten different anti-keratin studied cytokeratin (CK) expression in DC using ten different antibodies against CK1, 7, 8, 10, 14, 15, 16, 17, 18 and 19, anti-keratin antibodies against CK1, 7, 8, 10, 14, 15, 16, 17, and anti-filaggrin antibody. 18 and 19, and anti-filaggrin (filament aggregating protein) antibody. In the cyst wall of DC, CK1 and 10 were expressed Materials and methods in suprabasal layer, and CK14 was limited to the basal layer. In sebaceous gland-like structures, CK14 was detected in The patient, a 48-year-old male had DC located on the left sebaceous acinus, and CK17 was detected in sebaceous duct. postauriclar area from at birth. The other patient, a 31-year- The other CKs were not detected. Filaggrin was intensely old female developed DC on the right postauriclar area ten detected in the granular layer in the cyst wall of DC. -

Immunohistochemistry in Diagnosis of Soft Tissue Tumours Cyril Fisher
Immunohistochemistry in Diagnosis of Soft Tissue Tumours Cyril Fisher To cite this version: Cyril Fisher. Immunohistochemistry in Diagnosis of Soft Tissue Tumours. Histopathology, Wiley, 2010, 58 (7), pp.1001. 10.1111/j.1365-2559.2010.03707.x. hal-00613811 HAL Id: hal-00613811 https://hal.archives-ouvertes.fr/hal-00613811 Submitted on 6 Aug 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Histopathology Immunohistochemistry in Diagnosis of Soft Tissue Tumours ForJournal: Histopathology Peer Review Manuscript ID: HISTOP-08-10-0420 Wiley - Manuscript type: Review Date Submitted by the 01-Aug-2010 Author: Complete List of Authors: fisher, cyril; royal marsden hospital, histopathology Keywords: soft tissue tumours, immunohistochemistry, sarcoma, diagnosis Published on behalf of the British Division of the International Academy of Pathology Page 1 of 39 Histopathology Immunohistochemistry in Diagnosis of Soft Tissue Tumours For PeerCyril FisherReview Royal Marsden Hospital, London UK Correspondence to: Prof Cyril Fisher MD DSc FRCPath Dept of Histopathology The Royal Marsden Hospital 203 Fulham Road London SW3 6JJ UK Email: [email protected] Tel: +44 207 808 2631 Fax +44 207 808 2578 Running Title : Soft Tissue Tumour Immunohistochemistry Key Words Immunohistochemistry, sarcoma, diagnosis, soft tissue tumour 1 Published on behalf of the British Division of the International Academy of Pathology Histopathology Page 2 of 39 Abstract Immunohistochemistry in soft tissue tumours, and especially sarcomas, is used to identify differentiation in the neoplastic cells. -

HUMAN CYTOKERATINS Their Use As Targets in Cancer Management By
UMEÅ UNIVERSITY MEDICAL DISSERTATIONS New series No 284 — ISSN 0346-6612 From the Department of Medical Biochemistry and Biophysics University of Umeå, Umeå, Sweden HUMAN CYTOKERATINS their use as targets in cancer management by Birgitta Sundström Umeå 1990 HUMAN CYTOKERATINS their use as targets in cancer management AKADEMISK AVHANDLING som med vederbörigt tillstånd av Rektorsämbetet vid Umeå universitet för avläggande av doktorsexamen i medicinsk vetenskap kommer att offentligen försvaras i sal B, LU 0 (Naturvetarhuset), Umeå universitet, fredagen den 5 oktober 1990, kl. 09.15 av Birgitta Sundström Umeå 1990 ABSTRACT HUMAN CYTOKERATINS - their use as targets in cancer management Birgitta Sundström, Department of Medical Biochemistry and Biophysics, University of Umeå, S 901 87 Umeå, Sweden. Cytokeratins, biochemically related to intermediate filaments (IF), form an intracellular network of filaments which contributes to the mechanical stabilizing of the cell. 19 individual polypeptides, divided into two groups, constitute the cytokeratin family. Each type of epithelial cell can be characterized by its content of cytokeratin polypeptides since the expression pattern varies with the type of epithelium. During the transformation of epithelial cells into tumours, the cytokeratin patterns are usually maintained. This property has enabled cytokeratins to be used as tumour markers, especially for tumours not easily classified. In order to evaluate cytokeratins as tumour markers, we have generated a battery of monoclonal antibodies (MAbs) against cytokeratins extracted from carcinomas. Five antibodies were further characterized. All reacted with cytokeratin 8 (CK 8) amongst others, but only one, TS 1, was specific for this keratin. CK 8 is o ne of the most abundant keratins in carcinomas. -

Inducible Expression of Filaggrin Increases Keratinocyte Susceptibility to Apoptotic Cell Death
Cell Death and Differentiation (2000) 7, 566 ± 573 ã 2000 Macmillan Publishers Ltd All rights reserved 1350-9047/00 $15.00 www.nature.com/cdd Inducible expression of filaggrin increases keratinocyte susceptibility to apoptotic cell death MK Kuechle*1,2, RB Presland1,2, SP Lewis1, P Fleckman2 and diamine tetra-acetic acid; GFP, green ¯uorescent protein; PBS, BA Dale1,2,3,4 phosphate buffered saline; REK, rat epidermal keratinocyte; TBS, tris buffered saline; TUNEL, terminal deoxytransferase-mediated 1 Department of Oral Biology, University of Washington, Seattle, Washington, dUTP-biotin nick end labeling WA 98195, USA 2 Department of Medicine (Dermatology), University of Washington, Seattle, Washington, WA 98195, USA 3 Department of Periodontics, University of Washington, Seattle, Washington, Introduction WA 98195, USA The stratum corneum is the thin (12 ± 15 mm) tough, outer- 4 Department of Biochemistry, University of Washington, Seattle, Washington, WA 98195, USA most layer of the epidermis composed of overlapping, 1 * Corresponding author: MK Kuechle, Departments of Oral Biology/Medicine flattened, corneocytes and lipid-rich, intercellular lamellae. (Dermatology), Box 357132, University of Washington, Seattle, Washington, The stratum corneum functions as a barrier both to keep WA 98185-7132, USA. Tel: (206) 543-1595; Fax: (206) 685-3162; environmental insults out and to prevent water loss. Many E-mail: [email protected] morphologic and biochemical changes occur during the formation of the stratum corneum. Loricrin, involucrin, -

Degradation of Plectin with Modulation of Cytokeratin 18 In
in vivo 22 : 543-548 (2008) Degradation of Plectin with Modulation of Cytokeratin 18 in Human Liver Cells during Staurosporine- induced Apoptosis YI-HSIANG LIU 1,2,3* , CHIUNG-CHI CHENG 4* , CHIN-CHIN HO 5* , WEI-TING CHAO 6, REN-JENG PEI 1,3 , YUNG-HSIANG HSU 2, KUN-TU YEH 7, LU-CHANG HO 8, MING-CHUANG TSAI 3 and YIH-SHYONG LAI 3 1Department of Pathology, Jen Ai Hospital, Taichung; 2Department of Pathology, Tzu Chi Hospital and University, Hualien; Departments of 3Pathology and 8Surgery, Hospital and Medical College of Chung Shan Medical University, Taichung; 4Institute of Medicine of Chung Shan Medical University, Taichung; 5Department of Nursing, Central Taiwan University of Science and Technology, Taichung, Taiwan, R.O.C. ; 6Department of Molecular Physiology and Biophysics, Baylor College of Medicine, Houston, TX 77054, U.S.A.; 7Department of Pathology, Changhua Christian Hospital, Changhua, Taiwan, R.O.C. Abstract. Background: Hepatoma cells are morphologically constitute the largest family of the cytoskeleton (1). different from those of the normal liver. Intermediate Hepatocytes have a very simple CK composition and express filaments (IFs) are important in building the cellular only one CK pair, CK8 (type II) and CK18 (type I) (2). CKs architecture and maintaining the outline of cells. Plectin is are required for the maintenance of hepatocyte integrity (3) a cross-linking protein that organizes the cytoskeleton into a and the altered expression of CK genes is known to be related stable meshwork, which can maintain the uniform size and to liver diseases, including chronic hepatitis, increased shape of hepatocytes. Apoptosis might be the most possible hepatocyte fragility and decreased bile secretion (4). -

The Cytokeratin Filament-Aggregating Protein Filaggrin Is the Target of the So-Called "Antikeratin Antibodies," Autoantibodies Specific for Rheumatoid Arthritis
The cytokeratin filament-aggregating protein filaggrin is the target of the so-called "antikeratin antibodies," autoantibodies specific for rheumatoid arthritis. M Simon, … , G Salama, G Serre J Clin Invest. 1993;92(3):1387-1393. https://doi.org/10.1172/JCI116713. Research Article In rheumatoid arthritis (RA), the high diagnostic value of serum antibodies to the stratum corneum of rat esophagus epithelium has been widely reported. These so-called "antikeratin antibodies," detected by indirect immunofluorescence, were found to be autoantibodies since they also labeled human epidermis. Despite their name, the actual target of these autoantibodies was not known. In this study, a 40-kD protein (designated as 40K), extracted from human epidermis and specifically immunodetected by 75% of RA sera, was purified and identified as a neutral/acidic isoform of basic filaggrin, a cytokeratin filament-aggregating protein, by peptide mapping studies and by the following evidences: (a) mAbs specific for filaggrin reacted with the 40K protein; (b) the autoantibodies, affinity-purified from RA sera on the 40K protein, immunodetected purified filaggrin; (c) the reactivity of RA sera to the 40K protein was abolished after immunoadsorption with purified filaggrin; (d) the 40K protein and filaggrin had similar amino acid compositions. Furthermore, autoantibodies against the 40K protein and the so-called "antikeratin antibodies" were shown, by immunoadsorption experiments, to be largely the same. The identification of filaggrin as a RA-specific autoantigen could -

Cytoskeleton Markers
ptglab.com 1 CYTOSKELETON MARKERS www.ptglab.com Introduction The cytoskeleton is a three-dimensional network supporting and stabilizing the cell. All cells, even bacteria, have a type of cytoskeleton. It is responsible for the shape of the cell and its mechanical properties. Many dynamic cellular processes cooperate with the cytoskeleton, such as cell motion, cell division, intracellular transport, and cell signaling. Therefore, the cytoskeleton interacts with several cytoplasmic proteins or organelles. The cytoskeletal network is composed of three different protein structures named filaments: microtubules, microfilaments (actin), and intermediate filaments. These proteins form their own unique networks within the cell that have different interdependent functions. Main Functions of the Cytoskeleton Structural support Cell trafficking Transducer of mechanical signals Associated with several diseases Cellular signaling Cell Illustrating The Three Different Cytoskeleton Structure Proteins Please Note: All products featured in this catalog are for research use only. 2 Cytoskeleton Markers Most Popular Antibody Name Catalog Number Type Applications Cytoskeleton Markers ACTA2/alpha 5 23081-1-AP Rabbit Poly ELISA, IHC, IP, WB From Proteintech smooth muscle actin alpha Tubulin 4 11224-1-AP Rabbit Poly ELISA, FC, IF, IHC, IP, WB beta Actin 423 20536-1-AP Rabbit Poly ELISA, IF, IHC, WB beta Actin 399 60008-1-Ig Mouse Mono ELISA, FC, IF, IHC, WB beta Tubulin 11 10068-1-AP Rabbit Poly ELISA, IF, IHC, IP, WB Cofilin 5 10960-1-AP Rabbit Poly ELISA, IF, IHC, WB Cytokeratin 17 specific 17516-1-AP Rabbit Poly ELISA, FC, IF, IHC, IP, WB Desmin 2 60226-1-Ig Mouse Mono ELISA, IHC, WB GFAP 5 60190-1-Ig Mouse Mono ELISA, IF, IHC, IP, WB Palladin 5 10853-1-AP Rabbit Poly ELISA, FC, IF, IHC, IP, WB Vimentin 54 10366-1-AP Rabbit Poly ELISA, FC, IF, IHC, WB 00 This number shows the amount of times our antibody has been cited in a publication.