Keratin Expression During Early Embryonic Development of Bufo Bufo Gargarizans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In Situlocalization of Cytoskeletal Elements in the Human Trabecular
Investigative Ophthalmology & Visual Science. Vol. 31. No. 9. September 1990 Cops right £• Association lor Research in Vision and Ophthalmology In Situ Localization of Cytoskeletal Elements in the Human Trabecular Meshwork and Cornea Robert N. Weinreb* and Mark I. Ryderf The authors compared cytoskeletal elements of the in situ human trabccular-mcshwork cell with in situ human corneal cells using indirect immunofluorcsccncc staining for tubulin and intermediate filaments (vimentin, cytokeratin, and desmin) and NBD-phallacidin staining for f-actin using both fixed frozen and unfixed frozen sections from postmortem eyes. Both f-actin and tubulin were found throughout the cell body of trabecular-meshwork cells, keratocytes, corneal endothelium, and corneal epithelium. The f-actin staining pattern was concentrated at the cell periphery of these four cell types. Vimentin stain was intensely localized in focal areas of the trabecular-meshwork cell, keratocytes, and throughout the corneal cndothelium. A general anticytokeratin antibody was intensely localized in corneal epithelium and endothelium. However, PKK-1 anticytokeratin antibody was seen only in superficial layers of corneal epithelium and not in corneal endothelium. The 4.62 anticytokeratin antibody was not observed in either corneal epithelium or endothelium. None of these three cytokera- tin antibodies were seen in trabccular-mcshwork cells or keratocytes. Desmin stain was not noted in any of these cell types. In general, cytoskeletal staining of unfixed frozen sections showed a similar staining pattern for f-actin and tubulin but a more uniform and intense staining pattern for vimentin and cytokcratin compared with fixed frozen material. The authors conclude that these cytoskclctal stains can differentiate human Irabeciilar-meshwork cells from cells of the cornea in situ. -

Decreased Expression of Profilin 2 in Oral Squamous Cell Carcinoma and Its Clinicopathological Implications
ONCOLOGY REPORTS 26: 813-823, 2011 Decreased expression of profilin 2 in oral squamous cell carcinoma and its clinicopathological implications C.Y. MA1,2, C.P. ZHANG1,2, L.P. ZHONG1,2, H.Y. PAN1,2, W.T. CHEN1,2, L.Z. WANG3, O.W. ANDREW4, T. JI1 and W. HAN1,2 1Department of Oral and Maxillofacial Surgery, Ninth People's Hospital, College of Stomatology; 2Shanghai Key Laboratory of Stomatology and Shanghai Research Institute of Stomatology; 3Department of Oral Pathology, Ninth People's Hospital, College of Stomatology, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, P.R. China; 4Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, National University of Singapore, Singapore 119074, Singapore Received February 8, 2011; Accepted April 11, 2011 DOI: 10.3892/or.2011.1365 Abstract. Profilins are small proteins essential for many clinical and pathological significance. In conclusion, PFN2 normal cellular dynamics and constitute one of the crucial can be utilized as both a potential suppressor marker and a components of actin-based cellular motility. Several recent prognostic protein for OSCC. The function of PFN2 may be to studies have implicated a role for the profilin (PFN) family in regulate the N-WASP/Arp2/3 signaling pathway. cancer pathogenesis and progression. However, their expression and promising functions are largely unknown in oral squamous Introduction cell carcinoma (OSCC). In this study, we analyzed the correlation between PFN1 and PFN2 expression in vitro and Oral squamous cell carcinoma (OSCC) is a significant public in vivo. The protein expression levels were roughly compared health problem with >300,000 new cases being diagnosed between cell lines (HIOEC, HB96) with the employment of annually worldwide (1). -
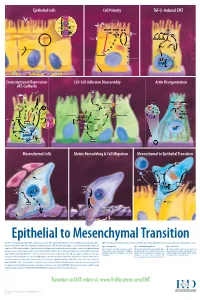
Epithelial to Mesenchymal Transition
Epithelial Cells Cell Polarity TGF-b-Induced EMT MUC-1 O-glycosylation Epithelial Cells ZO-1 Occludin Apical Membrane Tight F-Actin Microvilli Junction Claudin F-Actin p120 β-Catenin Adherens F-Actin Ezrin TGF-β dimer Junction E-Cadherin α-Catenin Plakophilin Crumbs Complex PAR Complex Desmocollin Desmoplakin Desmosome PtdIns(4,5)P2 TGF-β RII TGF-β RI CRB Cdc42Par6 Desmoglein Cytokeratin Pals1 PatJ Tight Junction Plakoglobin aPKC Par3 Domain Smad7 Extracellular PTEN JNK ERK1/2 p38 SARA Smurf1 Cortical Actin Cytoskeleton Space Par3 ZO-1 Adherens Junction PI 3-K Domain Smad-independent Signaling (–) Smad7 Translocation Smad2/3 PtdIns(3,4,5)P3 Smad4 Smad4 NEDD4 Cytokeratin Intermediate Filaments Smad2 Smad4 Smad3 LLGL Proteasome SCRIB DLG Scribble Complex Fibronectin Twist Smad2/3 Vitronectin ZEB 1/2 Microtubule Network Smad4 N-Cadherin Snail Basolateral Membrane CoA, Collagen I Slug CoR MMPs DNA-binding (+) Claudin Desmoplakin Transcription Factor Occludin Cytokeratins E-Cadherin Plakoglobin Integrins β α Nidogen-1/Entactin Perlecan Laminin Collagen IV Transcriptional Repression Cell-Cell Adhesion Disassembly Actin Reorganization of E-Cadherin TGF-β dimer EGF TGF-β RII TGF-β RI IGF FGF Receptor TNF-α Tyrosine Kinase Par6 TNF RI Apical Focal Adhesion Constriction Actin Depolymerization F-Actin Smurf1 Occludin Wnt Frizzled Myosin II Ras RhoA α-Actinin Myosin II ROCK AxinCK1 Dishevelled GSK-3 PI 3-K Src Zyxin MLC Phosphatase APC Proteasome FAK Vinculin RhoA ILK Talin (Inactive) Hakai Talin FAK F-Actin E-Cadherin LIMK Akt Paxillin FAK Stress -

U·M·I University Microfilms International a Bell & Howell Information Company 300 North Zeeb Road
The functional role(s) of dual intermediate filament expression in tumor cell migration and invasion. Item Type text; Dissertation-Reproduction (electronic) Authors Chu, Yi-Wen. Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 02/10/2021 04:10:29 Link to Item http://hdl.handle.net/10150/186143 INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. -

75 2. INTRODUCTION Triple-Negative Breast Cancer (TNBC)
[Frontiers in Bioscience, Scholar, 11, 75-88, March 1, 2019] The persisting puzzle of racial disparity in triple negative breast cancer: looking through a new lens Chakravarthy Garlapati1, Shriya Joshi1, Bikram Sahoo1, Shobhna Kapoor2, Ritu Aneja1 1Department of Biology, Georgia State University, Atlanta, GA, USA, 2Department of Chemistry, Indian Institute of Technology Bombay, Powai, India TABLE OF CONTENTS 1. Abstract 2. Introduction 3. Dissecting the TNBC racially disparate burden 3.1. Does race influence TNBC onset and progression? 3.2. Tumor microenvironment in TNBC and racial disparity 3.3. Differential gene signatures and pathways in racially distinct TNBC 3.4. Our Perspective: Looking racial disparity through a new lens 4. Conclusion 5. Acknowledgement 6. References 1. ABSTRACT 2. INTRODUCTION Triple-negative breast cancer (TNBC) Triple-negative breast cancer (TNBC), is characterized by the absence of estrogen a subtype of breast cancer (BC), accounts for and progesterone receptors and absence 15-20% of all BC diagnoses in the US. It has of amplification of human epidermal growth been recognized that women of African descent factor receptor (HER2). This disease has no are twice as likely to develop TNBC than approved treatment with a poor prognosis women of European descent (1). As the name particularly in African-American (AA) as foretells, TNBCs lack estrogen, progesterone, compared to European-American (EA) and human epidermal growth factor receptors. patients. Gene ontology analysis showed Unfortunately, TNBCs are defined by what they specific gene pathways that are differentially “lack” rather than what they “have” and thus this regulated and gene signatures that are negative nomenclature provides no actionable differentially expressed in AA as compared to information on “druggable” targets. -

Proteomic Expression Profile in Human Temporomandibular Joint
diagnostics Article Proteomic Expression Profile in Human Temporomandibular Joint Dysfunction Andrea Duarte Doetzer 1,*, Roberto Hirochi Herai 1 , Marília Afonso Rabelo Buzalaf 2 and Paula Cristina Trevilatto 1 1 Graduate Program in Health Sciences, School of Medicine, Pontifícia Universidade Católica do Paraná (PUCPR), Curitiba 80215-901, Brazil; [email protected] (R.H.H.); [email protected] (P.C.T.) 2 Department of Biological Sciences, Bauru School of Dentistry, University of São Paulo, Bauru 17012-901, Brazil; [email protected] * Correspondence: [email protected]; Tel.: +55-41-991-864-747 Abstract: Temporomandibular joint dysfunction (TMD) is a multifactorial condition that impairs human’s health and quality of life. Its etiology is still a challenge due to its complex development and the great number of different conditions it comprises. One of the most common forms of TMD is anterior disc displacement without reduction (DDWoR) and other TMDs with distinct origins are condylar hyperplasia (CH) and mandibular dislocation (MD). Thus, the aim of this study is to identify the protein expression profile of synovial fluid and the temporomandibular joint disc of patients diagnosed with DDWoR, CH and MD. Synovial fluid and a fraction of the temporomandibular joint disc were collected from nine patients diagnosed with DDWoR (n = 3), CH (n = 4) and MD (n = 2). Samples were subjected to label-free nLC-MS/MS for proteomic data extraction, and then bioinformatics analysis were conducted for protein identification and functional annotation. The three Citation: Doetzer, A.D.; Herai, R.H.; TMD conditions showed different protein expression profiles, and novel proteins were identified Buzalaf, M.A.R.; Trevilatto, P.C. -

Keratin 12-Deficient Mice Have Fragile Corneal Epithelia
Keratin 12-Deficient Mice Have Fragile Corneal Epithelia Winston W.—Y. Kao,* Chia-YangLiu,* Richard L. Converse,* Atsushi Shiraishi* Candace W.-C. Kao,* Masamichi Ishizaki* Thomas Doetschman,^ and John Duffy-f Purpose. Expression of the K3-K12 keratin pair characterizes the corneal epithelial differentia- tion. To elucidate the role of keratin 12 in the maintenance of corneal epithelium integrity, the authors bred mice deficient in keratin 12 by gene-targeting techniques. Methods. One allele of murine Krtl.12 gene was ablated in the embryonic stem cell line, E14.1, by homologous recombination with a DNA construct in which the DNA element between intron 2 and exon 8 of the keratin 12 gene was replaced by a neo-gene. The homologous recombinant embryonic stem cells were injected to mouse blastocysts, and germ lines of chimeras were obtained. The corneas of heterozygous and homozygous mice were characterized by clinical observations using stereomicroscopy, histology with light and electron microscopy, Western immunoblot analysis, immunohistochemistry, in situ hybridization, and Northern hybridization. Results. The heterozygous mice (+/—) one allele of the Krtl.12 gene appear normal and do not develop any clinical manifestations (e.g., corneal epithelial defects). Homozygous mice (—/—) develop normally and suffer mild corneal epithelial erosion. Their corneal epithelia are fragile and can be removed by gentle rubbing of the eyes or brushing with a Microsponge. The corneal epithelium of the homozygote (—/—) does not express keratin 12 as judged by immunohistochemis- try, Western immunoblot analysis with epitope-specific anti-keratin 12 antibodies, Nordiern hybrid- ization with 32P-labeled keratin 12 cDNA, and in situ hybridization with an anti-sense keratin 12 riboprobe. -

Keratin Remodelling in Stress Tan Tong San National
KERATIN REMODELLING IN STRESS TAN TONG SAN NATIONAL UNIVERSITY OF SINGAPORE 2012 i KERATIN REMODELLING IN STRESS TAN TONG SAN (B. Sc. (Hons.), NUS) A THESIS SUBMITTED FOR THE DEGREE OF DOCTOR OF PHILOSOPHY NUS Graduate School for Integrative Sciences and Engineering NATIONAL UNIVERSITY OF SINGAPORE 2012 ii iii ACKNOWLEDGEMENTS I would like to express my deepest gratitude to my supervisor, Prof. Birgit Lane, for her guidance and mentorship, and for giving me the opportunity, independence and resources to conduct my research. Her depth of knowledge and passion for scientific discovery have been a great source of inspiration and motivation for me throughout these four years of PhD endeavour, and will continue to be in the future. I would also like to thank my thesis advisory committee, Prof. Michael Raghunath and A/Prof. Edward Manser, for their critical feedback along the way. My sincere thanks and appreciation also go to John Common, for his insightful comments and suggestions throughout the course of this project. I would also like to acknowledge Ildiko for initiating the keratin phosphorylation project. Special thanks also go to Cedric, Chida, Darren, Gopi, Nama, John Lim, Delina, Chye Ling, Huijia and Declan, for their help in research techniques. I am most fortunate to be part of the EBL lab, a stimulating and lively place to work in. Thanks go to Kenneth, Zhou Fan, Paula, Chai Ling, Christine, Yi Zhen, Rosita, Vivien and Giorgiana, fellow students who join me in the pursuit of a doctorate. Appreciation is also extended to Iskandar, Jun Xian, Carol, Anita, Yi Ling, Ting Ting and other lab members for their support and help. -

Damage of Hair Follicle Stem Cells and Alteration of Keratin Expression in External Radiation-Induced Acute Alopecia
INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 30: 579-584, 2012 Damage of hair follicle stem cells and alteration of keratin expression in external radiation-induced acute alopecia NAOKI NANASHIMA, KOICHI ITO, TAKASHI ISHIKAWA, MANABU NAKANO and TOSHIYA NAKAMURA Department of Biomedical Sciences, Division of Medical Life Sciences, Hirosaki University Graduate School of Health Sciences, Hirosaki, Japan Received April 4, 2012; Accepted May 28, 2012 DOI: 10.3892/ijmm.2012.1018 Abstract. Alopecia is known as a symptom of acute radia- disturbances and blood and bone marrow disorders are known tion, yet little is known concerning the mechanism of this to occur within several hours to several weeks after 1-6 Gy of phenomenon and the alteration of hair protein profiles. To radiation exposure (4,6). examine this, 6-week-old male C57/BL6 mice were exposed Hair loss is also an effect of ARS, but little is known to 6 Gy of X-ray irradiation, which caused acute alopecia. about the mechanism underlying radiation-induced hair loss. Their hair and skin were collected, and hair proteins were In humans, hair loss is caused by radiation of more than analyzed with liquid chromatography/electrospray-ionization 3 Gy, and almost complete hair loss occurs within weeks of mass spectrometry and immunohistochemistry. No change exposure to 6 Gy (4,6). Since blood stem cells are sensitive to was observed in the composition of major hair keratins, such radiation (7), hair loss is thought to be caused by irradiation- as Krt81, Krt83 and Krt86. However, cytokeratin Krt15 and induced stem cell damage, yet no studies have investigated CD34, which are known as hair follicle stem cell markers, this hypothesis. -

Cytoskeletal Remodeling in Cancer
biology Review Cytoskeletal Remodeling in Cancer Jaya Aseervatham Department of Ophthalmology, University of Texas Health Science Center at Houston, Houston, TX 77054, USA; [email protected]; Tel.: +146-9767-0166 Received: 15 October 2020; Accepted: 4 November 2020; Published: 7 November 2020 Simple Summary: Cell migration is an essential process from embryogenesis to cell death. This is tightly regulated by numerous proteins that help in proper functioning of the cell. In diseases like cancer, this process is deregulated and helps in the dissemination of tumor cells from the primary site to secondary sites initiating the process of metastasis. For metastasis to be efficient, cytoskeletal components like actin, myosin, and intermediate filaments and their associated proteins should co-ordinate in an orderly fashion leading to the formation of many cellular protrusions-like lamellipodia and filopodia and invadopodia. Knowledge of this process is the key to control metastasis of cancer cells that leads to death in 90% of the patients. The focus of this review is giving an overall understanding of these process, concentrating on the changes in protein association and regulation and how the tumor cells use it to their advantage. Since the expression of cytoskeletal proteins can be directly related to the degree of malignancy, knowledge about these proteins will provide powerful tools to improve both cancer prognosis and treatment. Abstract: Successful metastasis depends on cell invasion, migration, host immune escape, extravasation, and angiogenesis. The process of cell invasion and migration relies on the dynamic changes taking place in the cytoskeletal components; actin, tubulin and intermediate filaments. This is possible due to the plasticity of the cytoskeleton and coordinated action of all the three, is crucial for the process of metastasis from the primary site. -

9.4 | Intermediate Filaments
354 9.4 | Intermediate Filaments The second of the three major cytoskeletal Microtubule elements to be discussed was seen in the electron microscope as solid, unbranched Intermediate filaments with a diameter of 10–12 nm. They were named in- filament termediate filaments (or IFs ). To date, intermediate filaments have only been identified in animal cells. Intermediate fila- ments are strong, flexible, ropelike fibers that provide mechani- cal strength to cells that are subjected to physical stress, Gold-labeled including neurons, muscle cells, and the epithelial cells that line anti-plectin the body’s cavities. Unlike microfilaments and microtubules, antibodies IFs are a chemically heterogeneous group of structures that, in Plectin humans, are encoded by approximately 70 different genes. The polypeptide subunits of IFs can be divided into five major classes based on the type of cell in which they are found (Table 9.2) as well as biochemical, genetic, and immunologic criteria. Figure 9.41 Cytoskeletal elements are connected to one another by We will restrict the present discussion to classes I-IV, which are protein cross-bridges. Electron micrograph of a replica of a small por- found in the construction of cytoplasmic filaments, and con- tion of the cytoskeleton of a fibroblast after selective removal of actin sider type V IFs (the lamins), which are present as part of the filaments. Individual components have been digitally colorized to assist inner lining of the nuclear envelope, in Section 12.2. visualization. Intermediate filaments (blue) are seen to be connected to IFs radiate through the cytoplasm of a wide variety of an- microtubules (red) by long wispy cross-bridges consisting of the fibrous imal cells and are often interconnected to other cytoskeletal protein plectin (green). -

UCLA Electronic Theses and Dissertations
UCLA UCLA Electronic Theses and Dissertations Title Proteomic Analysis of Cancer Cell Metabolism Permalink https://escholarship.org/uc/item/8t36w919 Author Chai, Yang Publication Date 2013 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA Los Angeles Proteomic Analysis of Cancer Cell Metabolism A thesis submitted in partial satisfaction of the requirements of the degree Master of Science in Oral Biology by Yang Chai 2013 ABSTRACT OF THESIS Proteomic Analysis of Cancer Cell Metabolism by Yang Chai Master of Science in Oral Biology University of California, Los Angeles, 2013 Professor Shen Hu, Chair Tumor cells can adopt alternative metabolic pathways during oncogenesis. This is an event characterized by an enhanced utilization of glucose for rapid synthesis of macromolecules such as nucleotides, lipids and proteins. This phenomenon was also known as the ‘Warburg effect’, distinguished by a shift from oxidative phosphorylation to increased aerobic glycolysis in many types of cancer cells. Increased aerobic glycolysis was also indicated with enhanced lactate production and glutamine consumption, and has been suggested to confer growth advantage for proliferating cells during oncogenic transformation. Development of a tracer-based ii methodology to determine de novo protein synthesis by tracing metabolic pathways from nutrient utilization may certainly enhance current understanding of nutrient gene interaction in cancer cells. We hypothesized that the metabolic phenotype of cancer cells as characterized by nutrient utilization for protein synthesis is significantly altered during oncogenesis, and 13C stable isotope tracers may incorporate 13C into non-essential amino acids of protein peptides during de novo protein synthesis to reflect the underlying mechanisms in cancer cell metabolism.