Cytoskeleton Markers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
The Desmoplakin Carboxyl Terminus Coaligns with and Specifically Disrupts Intermediate Filament Networks When Expressed in Cultured Cells Thaddeus S
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central The Desmoplakin Carboxyl Terminus Coaligns with and Specifically Disrupts Intermediate Filament Networks When Expressed in Cultured Cells Thaddeus S. Stappenbeck and Kathleen J. Green Department of Pathology and the Cancer Center, Northwestern University Medical School, Chicago, Illinois 60611 Abstract. Specific interactions between desmoplakins tides including the 90-kD carboxy-terminal globular I and 11 (DP I and II) and other desmosomal or cyto- domain of DP I specifically colocalized with and ulti- skeletal molecules have been difficult to determine in mately resulted in the complete disruption of IF in part because of the complexity and insolubility of the both cell lines. This effect was specific for IF as micro- desmosome and its constituents . We have used a mo- tubule and microfilament networks were unaltered . lecular genetic approach to investigate the role that This effect was also specific for the carboxyl terminus DP I and 11 may play in the association of the desmo- of DP, as the expression of the 95-kD rod domain of somal plaque with cytoplasmic intermediate filaments DP I did not visibly alter IF networks. Immunogold (IF) . A series of mammalian expression vectors en- localization of COS-7 cells transfected with constructs coding specific predicted domains of DP I were tran- including the carboxyl terminus of DP demonstrated siently expressed in cultured cells that form (COS-7) an accumulation of mutant protein in perinuclear aggre- and do not form (NIH-3T3) desmosomes. Sequence gates within which IF subunits were sequestered. -

In Situlocalization of Cytoskeletal Elements in the Human Trabecular
Investigative Ophthalmology & Visual Science. Vol. 31. No. 9. September 1990 Cops right £• Association lor Research in Vision and Ophthalmology In Situ Localization of Cytoskeletal Elements in the Human Trabecular Meshwork and Cornea Robert N. Weinreb* and Mark I. Ryderf The authors compared cytoskeletal elements of the in situ human trabccular-mcshwork cell with in situ human corneal cells using indirect immunofluorcsccncc staining for tubulin and intermediate filaments (vimentin, cytokeratin, and desmin) and NBD-phallacidin staining for f-actin using both fixed frozen and unfixed frozen sections from postmortem eyes. Both f-actin and tubulin were found throughout the cell body of trabecular-meshwork cells, keratocytes, corneal endothelium, and corneal epithelium. The f-actin staining pattern was concentrated at the cell periphery of these four cell types. Vimentin stain was intensely localized in focal areas of the trabecular-meshwork cell, keratocytes, and throughout the corneal cndothelium. A general anticytokeratin antibody was intensely localized in corneal epithelium and endothelium. However, PKK-1 anticytokeratin antibody was seen only in superficial layers of corneal epithelium and not in corneal endothelium. The 4.62 anticytokeratin antibody was not observed in either corneal epithelium or endothelium. None of these three cytokera- tin antibodies were seen in trabccular-mcshwork cells or keratocytes. Desmin stain was not noted in any of these cell types. In general, cytoskeletal staining of unfixed frozen sections showed a similar staining pattern for f-actin and tubulin but a more uniform and intense staining pattern for vimentin and cytokcratin compared with fixed frozen material. The authors conclude that these cytoskclctal stains can differentiate human Irabeciilar-meshwork cells from cells of the cornea in situ. -

Actin Cytoskeleton of Spread Fibroblasts Appears to Assemble at the Cell Edges
J. Cell Sd. 82, 235-248 (1986) 235 Printed in Great Britain © The Company of Biologists Limited 1986 ACTIN CYTOSKELETON OF SPREAD FIBROBLASTS APPEARS TO ASSEMBLE AT THE CELL EDGES TATJANA M. SVITKINA, ALEXANDER A. NEYFAKH, JR Laboratory of Molecular Biology and Bioorganic Chemistry, Moscow State University, Moscow 119899, USSR AND ALEXANDER D. BERSHADSKY All-Union Cancer Research Center, Academy of Medical Sciences, Moscow 115478, USSR SUMMARY The action of metabolic inhibitors on actin cytoskeleton of cultured quail embryo fibroblasts has been studied using electron microscopy of platinum replicas and immunofluorescence microscopy. Sodium azide as well as other inhibitors (oligomycin and dinitrophenol) caused the disassembly of all types of actin structures: actin meshwork at the cell active edges, microfilament sheath underlying the cell surface, and microfilament bundles. Studying the time- and dose-dependence of the destruction process we have found that the active edge meshwork and microfilament sheath are much more labile than microfilament bundles. After the removal of metabolic inhibitors actin cytoskeleton restoration begins at the cell edges. The first sign of this process is the formation of actin meshwork along the whole cell perimeter (l-10min of recovery). Sometimes fragments of this meshwork bend upwards forming ruffles. Later (10-20 min of recovery) the microfilament sheath appears at the cell periphery as a narrow band. The sheath seems to be formed from the edge meshwork, since ruffles in the process of transformation to sheath could be seen. During the following restoration the microfilament sheath gradually expands towards the cell centre. The last step of actin cytoskeleton restoration (60—120 min of recovery) is the formation of bundles. -

Decreased Expression of Profilin 2 in Oral Squamous Cell Carcinoma and Its Clinicopathological Implications
ONCOLOGY REPORTS 26: 813-823, 2011 Decreased expression of profilin 2 in oral squamous cell carcinoma and its clinicopathological implications C.Y. MA1,2, C.P. ZHANG1,2, L.P. ZHONG1,2, H.Y. PAN1,2, W.T. CHEN1,2, L.Z. WANG3, O.W. ANDREW4, T. JI1 and W. HAN1,2 1Department of Oral and Maxillofacial Surgery, Ninth People's Hospital, College of Stomatology; 2Shanghai Key Laboratory of Stomatology and Shanghai Research Institute of Stomatology; 3Department of Oral Pathology, Ninth People's Hospital, College of Stomatology, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, P.R. China; 4Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, National University of Singapore, Singapore 119074, Singapore Received February 8, 2011; Accepted April 11, 2011 DOI: 10.3892/or.2011.1365 Abstract. Profilins are small proteins essential for many clinical and pathological significance. In conclusion, PFN2 normal cellular dynamics and constitute one of the crucial can be utilized as both a potential suppressor marker and a components of actin-based cellular motility. Several recent prognostic protein for OSCC. The function of PFN2 may be to studies have implicated a role for the profilin (PFN) family in regulate the N-WASP/Arp2/3 signaling pathway. cancer pathogenesis and progression. However, their expression and promising functions are largely unknown in oral squamous Introduction cell carcinoma (OSCC). In this study, we analyzed the correlation between PFN1 and PFN2 expression in vitro and Oral squamous cell carcinoma (OSCC) is a significant public in vivo. The protein expression levels were roughly compared health problem with >300,000 new cases being diagnosed between cell lines (HIOEC, HB96) with the employment of annually worldwide (1). -

HTS-Tubulin Polymerization Assay Biochem Kit™
The Protein Manual Experts Cytoskeleton, Inc. V 8.0 HTS-Tubulin Polymerization Assay Biochem Kit™ (>97% pure tubulin, Porcine Tubulin) Cat. # BK004P Phone: (303) 322.2254 Fax: (303) 322.2257 Customer Service: [email protected] cytoskeleton.com Technical Support: [email protected] cytoskeleton.com Page 2 Manual Contents Section I: Introduction About Tubulin -------------------------------------------------------------------------- 5 About the BK004P polymerization Assay -------------------------------------- 6-7 Comparison of Polymerization Assays ----------------------------------------- 8-9 Section II: Purchaser Notification ------------------------------------------------------------ 10 Section III: Kit Contents ------------------------------------------------------------------------- 11-12 Section V: Reconstitution and Storage of Components ----------------------------- 13 Section IV: Important Technical Notes Notes on Updated version --------------------------------------------------------- 14 Spectrophotometer settings ------------------------------------------------------- 14 Spectrophotometer pathlength---------------------------------------------------- 15 Temperature & time dependence of polymerization ------------------------ 15 Recommended pipetting technique --------------------------------------------- 15-16 Tubulin protein stability ------------------------------------------------------------- 16 Test compound or protein preparation ------------------------------------------ 16-17 Section VI: Assay Protocol -
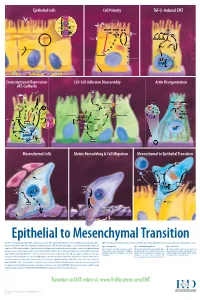
Epithelial to Mesenchymal Transition
Epithelial Cells Cell Polarity TGF-b-Induced EMT MUC-1 O-glycosylation Epithelial Cells ZO-1 Occludin Apical Membrane Tight F-Actin Microvilli Junction Claudin F-Actin p120 β-Catenin Adherens F-Actin Ezrin TGF-β dimer Junction E-Cadherin α-Catenin Plakophilin Crumbs Complex PAR Complex Desmocollin Desmoplakin Desmosome PtdIns(4,5)P2 TGF-β RII TGF-β RI CRB Cdc42Par6 Desmoglein Cytokeratin Pals1 PatJ Tight Junction Plakoglobin aPKC Par3 Domain Smad7 Extracellular PTEN JNK ERK1/2 p38 SARA Smurf1 Cortical Actin Cytoskeleton Space Par3 ZO-1 Adherens Junction PI 3-K Domain Smad-independent Signaling (–) Smad7 Translocation Smad2/3 PtdIns(3,4,5)P3 Smad4 Smad4 NEDD4 Cytokeratin Intermediate Filaments Smad2 Smad4 Smad3 LLGL Proteasome SCRIB DLG Scribble Complex Fibronectin Twist Smad2/3 Vitronectin ZEB 1/2 Microtubule Network Smad4 N-Cadherin Snail Basolateral Membrane CoA, Collagen I Slug CoR MMPs DNA-binding (+) Claudin Desmoplakin Transcription Factor Occludin Cytokeratins E-Cadherin Plakoglobin Integrins β α Nidogen-1/Entactin Perlecan Laminin Collagen IV Transcriptional Repression Cell-Cell Adhesion Disassembly Actin Reorganization of E-Cadherin TGF-β dimer EGF TGF-β RII TGF-β RI IGF FGF Receptor TNF-α Tyrosine Kinase Par6 TNF RI Apical Focal Adhesion Constriction Actin Depolymerization F-Actin Smurf1 Occludin Wnt Frizzled Myosin II Ras RhoA α-Actinin Myosin II ROCK AxinCK1 Dishevelled GSK-3 PI 3-K Src Zyxin MLC Phosphatase APC Proteasome FAK Vinculin RhoA ILK Talin (Inactive) Hakai Talin FAK F-Actin E-Cadherin LIMK Akt Paxillin FAK Stress -

Plakoglobin Is Required for Effective Intermediate Filament Anchorage to Desmosomes Devrim Acehan1, Christopher Petzold1, Iwona Gumper2, David D
ORIGINAL ARTICLE Plakoglobin Is Required for Effective Intermediate Filament Anchorage to Desmosomes Devrim Acehan1, Christopher Petzold1, Iwona Gumper2, David D. Sabatini2, Eliane J. Mu¨ller3, Pamela Cowin2,4 and David L. Stokes1,2,5 Desmosomes are adhesive junctions that provide mechanical coupling between cells. Plakoglobin (PG) is a major component of the intracellular plaque that serves to connect transmembrane elements to the cytoskeleton. We have used electron tomography and immunolabeling to investigate the consequences of PG knockout on the molecular architecture of the intracellular plaque in cultured keratinocytes. Although knockout keratinocytes form substantial numbers of desmosome-like junctions and have a relatively normal intercellular distribution of desmosomal cadherins, their cytoplasmic plaques are sparse and anchoring of intermediate filaments is defective. In the knockout, b-catenin appears to substitute for PG in the clustering of cadherins, but is unable to recruit normal levels of plakophilin-1 and desmoplakin to the plaque. By comparing tomograms of wild type and knockout desmosomes, we have assigned particular densities to desmoplakin and described their interaction with intermediate filaments. Desmoplakin molecules are more extended in wild type than knockout desmosomes, as if intermediate filament connections produced tension within the plaque. On the basis of our observations, we propose a particular assembly sequence, beginning with cadherin clustering within the plasma membrane, followed by recruitment of plakophilin and desmoplakin to the plaque, and ending with anchoring of intermediate filaments, which represents the key to adhesive strength. Journal of Investigative Dermatology (2008) 128, 2665–2675; doi:10.1038/jid.2008.141; published online 22 May 2008 INTRODUCTION dense plaque that is further from the membrane and that Desmosomes are large macromolecular complexes that mediates the binding of intermediate filaments. -

Transiently Structured Head Domains Control Intermediate Filament Assembly
Transiently structured head domains control intermediate filament assembly Xiaoming Zhoua, Yi Lina,1, Masato Katoa,b,c, Eiichiro Morid, Glen Liszczaka, Lillian Sutherlanda, Vasiliy O. Sysoeva, Dylan T. Murraye, Robert Tyckoc, and Steven L. McKnighta,2 aDepartment of Biochemistry, University of Texas Southwestern Medical Center, Dallas, TX 75390; bInstitute for Quantum Life Science, National Institutes for Quantum and Radiological Science and Technology, 263-8555 Chiba, Japan; cLaboratory of Chemical Physics, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892-0520; dDepartment of Future Basic Medicine, Nara Medical University, 840 Shijo-cho, Kashihara, Nara, Japan; and eDepartment of Chemistry, University of California, Davis, CA 95616 Contributed by Steven L. McKnight, January 2, 2021 (sent for review October 30, 2020; reviewed by Lynette Cegelski, Tatyana Polenova, and Natasha Snider) Low complexity (LC) head domains 92 and 108 residues in length are, IF head domains might facilitate filament assembly in a manner respectively, required for assembly of neurofilament light (NFL) and analogous to LC domain function by RNA-binding proteins in the desmin intermediate filaments (IFs). As studied in isolation, these IF assembly of RNA granules. head domains interconvert between states of conformational disor- IFs are defined by centrally located α-helical segments 300 to der and labile, β-strand–enriched polymers. Solid-state NMR (ss-NMR) 350 residues in length. These central, α-helical segments are spectroscopic studies of NFL and desmin head domain polymers re- flanked on either end by head and tail domains thought to be veal spectral patterns consistent with structural order. -

Neurofilaments: Neurobiological Foundations for Biomarker Applications
Neurofilaments: neurobiological foundations for biomarker applications Arie R. Gafson1, Nicolas R. Barthelmy2*, Pascale Bomont3*, Roxana O. Carare4*, Heather D. Durham5*, Jean-Pierre Julien6,7*, Jens Kuhle8*, David Leppert8*, Ralph A. Nixon9,10,11,12*, Roy Weller4*, Henrik Zetterberg13,14,15,16*, Paul M. Matthews1,17 1 Department of Brain Sciences, Imperial College, London, UK 2 Department of Neurology, Washington University School of Medicine, St Louis, MO, USA 3 a ATIP-Avenir team, INM, INSERM , Montpellier university , Montpellier , France. 4 Clinical Neurosciences, Faculty of Medicine, University of Southampton, Southampton General Hospital, Southampton, United Kingdom 5 Department of Neurology and Neurosurgery, Montreal Neurological Institute, McGill University, Montreal, Québec, Canada 6 Department of Psychiatry and Neuroscience, Laval University, Quebec, Canada. 7 CERVO Brain Research Center, 2601 Chemin de la Canardière, Québec, QC, G1J 2G3, Canada 8 Neurologic Clinic and Policlinic, Departments of Medicine, Biomedicine and Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland. 9 Center for Dementia Research, Nathan Kline Institute, Orangeburg, NY, 10962, USA. 10Departments of Psychiatry, New York University School of Medicine, New York, NY, 10016, 11 Neuroscience Institute, New York University School of Medicine, New York, NY, 10016, USA. 12Department of Cell Biology, New York University School of Medicine, New York, NY, 10016, USA 13 University College London Queen Square Institute of Neurology, London, UK 14 UK Dementia Research Institute at University College London 15 Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, the Sahlgrenska Academy at the University of Gothenburg, Mölndal, Sweden 16 Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden 17 UK Dementia Research Institute at Imperial College, London * Co-authors ordered alphabetically Address for correspondence: Prof. -

Intermediate Filament Accumulation Can Stabilize Microtubules in Caenorhabditis Elegans Motor Neurons
Intermediate filament accumulation can stabilize microtubules in Caenorhabditis elegans motor neurons Naina Kurupa, Yunbo Lia, Alexandr Goncharova, and Yishi Jina,b,1 aNeurobiology Section, Division of Biological Sciences, University of California, San Diego, La Jolla, CA 92093; and bDepartment of Cellular and Molecular Medicine, University of California, San Diego, La Jolla, CA 92093 Edited by H. Robert Horvitz, Massachusetts Institute of Technology, Cambridge, MA, and approved February 11, 2018 (received for review December 21, 2017) Neural circuits utilize a coordinated cellular machinery to form and Results eliminate synaptic connections, with the neuronal cytoskeleton Identification of IF Genes That Regulate Synapse Rewiring. At the playing a prominent role. During larval development of Caenorhabditis end of larval stage 1 (L1), the dorsal D (DD)-type motor neurons elegans, synapses of motor neurons are stereotypically rewired rewire their presynaptic connections from the ventral nerve cord through a process facilitated by dynamic microtubules (MTs). Through a (VNC) to the dorsal nerve cord (DNC), concurrent with the genetic suppressor screen on mutant animals that fail to rewire synap- birth of ventral D (VD)-type motor neurons, which then form ses, and in combination with live imaging and ultrastructural studies, synapses along the VNC (19). We visualized DD-neuron pre- we find that intermediate filaments (IFs) stabilize MTs to prevent syn- synaptic terminals using a GFP-tagged synaptobrevin (SNB- apse rewiring. Genetic ablation of IFs or pharmacological disruption of 1::GFP) reporter (juIs137:Pflp-13 SNB-1::GFP). In L1 animals, IF networks restores MT growth and rescues synapse rewiring defects discrete synaptic puncta were present along the ventral neurites in the mutant animals, indicating that IF accumulation directly alters MT (18), but in late larvae and adults, synaptic puncta were only seen stability. -

Cytoskeleton Cytoskeleton
CYTOSKELETON CYTOSKELETON The cytoskeleton is composed of three principal types of protein filaments: actin filaments, intermediate filaments, and microtubules, which are held together and linked to subcellular organelles and the plasma membrane by a variety of accessory proteins Muscle Contraction • Skeletal muscles are bundles of muscle fibers • Most of the cytoplasm consists of myofibrils, which are cylindrical bundles of two types of filaments: thick filaments of myosin (about 15 run in diameter) and thin filaments of actin (about 7 nm in diameter). • Each myofibril is organized as a chain of contractile units called sarcomeres, which are responsible for the striated appearance of skeletal and cardiac muscle. Structure of muscle cells Sarcomere • The ends of each sarcomere are defined by the Z disc. • Within each sarcomere, dark bands (called A bands because they are anisotropic when viewed with polarized light) alternate with light bands (called I bands for isotropic). • The I bands contain only thin (actin) filaments, whereas the A bands contain thick (myosin) filaments. • The myosin and actin filaments overlap in peripheral regions of the A band, whereas a middle region (called the H zone) contains only myosin. Muscle contraction • The basis for understanding muscle contraction is the sliding filament model, first proposed in 1954 both by Andrew Huxley and Ralph Niedergerke and by Hugh Huxley and Jean Hanson • During muscle contraction each sarcomere shortens, bringing the Z discs closer together. • There is no change in the width of the A band, but both the I bands and the H zone almost completely disappear. • These changes are explained by the actin and myosin filaments sliding past one another so that the actin filaments move into the A band and H zone. -

The Relationship Between Intermediate Filaments and Microfilaments Before and During the Formation of Desmosomes and Adherens-Ty
Published May 1, 1987 The Relationship between Intermediate Filaments and Microfilaments before and during the Formation of Desmosomes and Adherens-type Junctions in Mouse Epidermal Keratinocytes Kathleen J. Green, Benjamin Geiger,* Jonathan C. R. Jones, John C. Talian, and Robert D. Goldman Department of Cell Biology and Anatomy, Northwestern University Medical School, Chicago, Illinois 60611; and * Department of Chemical Immunology, The Weizmann Institute of Science, Rehovot, Israel Abstract. Actin, keratin, vinculin and desmoplakin ermost of the concentric MFB. Individual IF often organization were studied in primary mouse keratino- splay out, becoming interwoven into these MFB in the cytes before and during Ca2+-induced cell contact forma- region of cell-substrate contact. In the first 30 min af- tion. Double-label fluorescence shows that in cells cul- ter the Ca 2+ switch, areas of submembranous dense Downloaded from tured in low Ca 2÷ medium, keratin-containing inter- material (identified as adherens junctions), which are mediate filament bundles (IFB) and desmoplakin- associated with the perpendicular MFB, can be seen at containing spots are both concentrated towards the cell newly formed cell-ceU contact sites. By 1-2 h, IFB- center in a region bounded by a series of concentric desmosomal component complexes are aligned with microfilament bundles (MFB). Within 5-30 min after the perpendicular MFB as the complexes become jcb.rupress.org raising Ca 2+ levels, a discontinuous actin/vinculin-rich, redistributed to cell-cell interfaces. Cytochalasin D submembranous zone of fluorescence appears at cell- treatment causes the redistribution of actin into numer- cell interfaces. This zone is usually associated with ous patches; keratin-containing Lr:B undergo a con- short, perpendicular MFB, which become wider and comitant redistribution, forming foci that coincide with longer with time.