Section 3.7 Marine Mammals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

San Diego & Surrounding Areas
Welcome Welcome to the University of San Diego! We are happy you are here and we hope that you will soon come to look upon our campus as your second home. Your first three weeks will be very busy. This is normal for anyone coming to live and study in the United States. Cultural diversity is welcomed in our country and on our campus. We hope that you will find both your course of study at USD and the opportunity to engage in cultural exchange to be rewarding and satisfying experiences. This handbook is designed to provide you with information you need to make the transition from your country to the United States a little easier. If you have questions, please visit us at the Office of International Students and Scholars (OISS). We are here to help you. We wish you every success in your academic, social, and cultural endeavors. The OISS Team TABLE OF CONTENTS OISS SERVICES……………………………………………………………………………...3 Check-in / Immigration.…………………………………..…………………4 How to Stay “in Status”....…………………………………………………..6 Communications…………………………………………………..............................9 Mobile Phones….………………………………………………………………9 Local Mobile Phone Companies…...….……………………………...11 Making Overseas Phone Calls………………………………………..…12 Internet Connection………………………………………………………...12 Technical Support...………………………………………………..……...13 Mail/Shipping…….…………………………………………………………...13 Transportation……………………………………………………………………………..14 Bus/Trolley Information..……...……………………………………….. 14 Campus Tram.…………………………………………….......................16 USD Parking Permits……...………..…………………………………....17 Car -
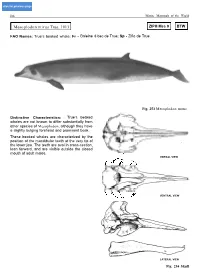
Mesoplodon Mirus True, 1913 ZIPH Mes 9 BTW
click for previous page 106 Marine Mammals of the World Mesoplodon mirus True, 1913 ZIPH Mes 9 BTW FAO Names: True’s beaked whale: Fr - Baleine à bec de True; Sp - Zifio de True. Fig. 253 Mesoplodon mirus Distinctive Characteristics: True’s beaked whales are not known to differ substantially from other species of Mesoplodon, although they have a slightly bulging forehead and prominent beak. These beaked whales are characterized by the position of the mandibular teeth at the very tip of the lower jaw. The teeth are oval in cross-section, lean forward, and are visible outside the closed mouth of adult males. DORSAL VIEW VENTRAL VIEW lLATERAL VIEWl Fig. 254 Skull Cetacea - Odontoceti - Ziphiidae 107 Can be confused with: At sea, True’s beaked whales are difficult to distinguish from other species of Mesoplodon (starting on p. 90). The only other species in which males have oval teeth at the tip of the lower jaw is Longman’s beaked whale (p. 112); whose appearance is not known. Size: Both sexes are known to reach lengths of slightly over 5 m. Weights of up to 1 400 kg have been recorded. Newborns are probably between 2 and 2.5 m. Geographical Distribution: True’s beaked whales are known only from strandings in Great Britain, from Florida to Nova Scotia in the North Atlantic, and from southeast Africa and southern Australia in the Indo-PacificcOcean. Fig. 255 Biology and Behaviour: There is almost no information available on the natural history of this species of beaked whale. Stranded animals have had squid in their stomachs. -

Chemical Composition and Product Quality Control of Turmeric
Stephen F. Austin State University SFA ScholarWorks Faculty Publications Agriculture 2011 Chemical composition and product quality control of turmeric (Curcuma longa L.) Shiyou Li Stephen F Austin State University, Arthur Temple College of Forestry and Agriculture, [email protected] Wei Yuan Stephen F Austin State University, Arthur Temple College of Forestry and Agriculture, [email protected] Guangrui Deng Ping Wang Stephen F Austin State University, Arthur Temple College of Forestry and Agriculture, [email protected] Peiying Yang See next page for additional authors Follow this and additional works at: http://scholarworks.sfasu.edu/agriculture_facultypubs Part of the Natural Products Chemistry and Pharmacognosy Commons, and the Pharmaceutical Preparations Commons Tell us how this article helped you. Recommended Citation Li, Shiyou; Yuan, Wei; Deng, Guangrui; Wang, Ping; Yang, Peiying; and Aggarwal, Bharat, "Chemical composition and product quality control of turmeric (Curcuma longa L.)" (2011). Faculty Publications. Paper 1. http://scholarworks.sfasu.edu/agriculture_facultypubs/1 This Article is brought to you for free and open access by the Agriculture at SFA ScholarWorks. It has been accepted for inclusion in Faculty Publications by an authorized administrator of SFA ScholarWorks. For more information, please contact [email protected]. Authors Shiyou Li, Wei Yuan, Guangrui Deng, Ping Wang, Peiying Yang, and Bharat Aggarwal This article is available at SFA ScholarWorks: http://scholarworks.sfasu.edu/agriculture_facultypubs/1 28 Pharmaceutical Crops, 2011, 2, 28-54 Open Access Chemical Composition and Product Quality Control of Turmeric (Curcuma longa L.) ,1 1 1 1 2 3 Shiyou Li* , Wei Yuan , Guangrui Deng , Ping Wang , Peiying Yang and Bharat B. Aggarwal 1National Center for Pharmaceutical Crops, Arthur Temple College of Forestry and Agriculture, Stephen F. -

California Sea Lion Interaction and Depredation Rates with the Commercial Passenger Fishing Vessel Fleet Near San Diego
HANAN ET AL.: SEA LIONS AND COMMERCIAL PASSENGER FISHING VESSELS CalCOFl Rep., Vol. 30,1989 CALIFORNIA SEA LION INTERACTION AND DEPREDATION RATES WITH THE COMMERCIAL PASSENGER FISHING VESSEL FLEET NEAR SAN DIEGO DOYLE A. HANAN, LISA M. JONES ROBERT B. READ California Department of Fish and Game California Department of Fish and Game c/o Southwest Fisheries Center 1350 Front Street, Room 2006 P.O. Box 271 San Diego, California 92101 La Jolla, California 92038 ABSTRACT anchovies, Engraulis movdax, which are thrown California sea lions depredate sport fish caught from the fishing boat to lure surface and mid-depth by anglers aboard commercial passenger fishing fish within casting range. Although depredation be- vessels. During a statewide survey in 1978, San havior has not been observed along the California Diego County was identified as the area with the coast among other marine mammals, it has become highest rates of interaction and depredation by sea a serious problem with sea lions, and is less serious lions. Subsequently, the California Department of with harbor seals. The behavior is generally not Fish and Game began monitoring the rates and con- appreciated by the boat operators or the anglers, ducting research on reducing them. The sea lion even though some crew and anglers encourage it by interaction and depredation rates for San Diego hand-feeding the sea lions. County declined from 1984 to 1988. During depredation, sea lions usually surface some distance from the boat, dive to swim under RESUMEN the boat, take a fish, and then reappear a safe dis- Los leones marinos en California depredan peces tance away to eat the fish, tear it apart, or just throw colectados por pescadores a bordo de embarca- it around on the surface of the water. -

The Navy's Environmental Program in the Southwest; Challenges for FY20+
NAVFAC SOUTHWEST The Navy’s Environmental Program in the Southwest; Challenges for FY20+ San Diego Environmental Professionals (SDEP) 12 November 2019 Derral Van Winkle, PG Environmental Remediation PLL NAVFAC Southwest NAVFAC SW Leadership and Management • Commanding Officer -- CAPT OESTERICHER −Executive Officer – CAPT Jeffery Powell −Business Director – Kathy Stewart −Operations Officer – CAPT Laurie Scott • Environmental BLL / N45 Shore EV Program Manager – Brian Gordon −EV1 Env. Compliance PLL -- Kathie Beverly −EV2 Env. Planning and Conservation PLL -- Connie Moen (acting) −EV3 Env. Restoration PLL -- Derral Van Winkle −EV4 Env. Resources and Assessment PLL -- Delphine Lee Notes: BLL – Business Line Leader PLL – Product Line Leader 2 NAVFAC Southwest 11/11/2019 Navy Region Southwest NRSW by the numbers: Naval Air Station Fallon • 10 Installations Naval • 189 Special Areas Support Naval Air Station Lemoore • 11,000 buildings / structures Activity • 42 Piers / Wharves Monterey • 19 Runways Naval Air Weapons Station China • 61 Hangars Lake • 1.8 M acres Naval Base Ventura County •Port Hueneme Naval Weapons Station Seal Beach • $800M annual budget •Point Mugu •Det Corona • 10,000 employees •San Nicolas Island (SNI) •Det Fallbrook •Fort Hunter Liggett • 325,000 customers Naval Base Point Loma • 17 NOSCs •SUBASE • 8 Small Arms Ranges •Old Town Complex •Harbor Drive Annex Naval Air Facility El Centro NAVAL Base Coronado Naval Base San Diego •NASNI •Broadway Complex •NAB •Naval Medical Center San Diego •NOLF Imperial Beach •NALF San Clemente -

Navy Gulf of Alaska Testing and Training 2017 Rule Application
REQUEST FOR LETTER OF AUTHORIZATION FOR THE INCIDENTAL HARASSMENT OF MARINE MAMMALS RESULTING FROM U.S. NAVY TRAINING ACTIVITIES IN THE GULF OF ALASKA TEMPORARY MARITIME ACTIVITIES AREA Submitted to: Office of Protected Resources National Marine Fisheries Service 1315 East-West Highway Silver Spring, Maryland 20910-3226 Submitted by: Commander, United States Pacific Fleet 250 Makalapa Drive Pearl Harbor, Hawaii 96860-3131 Revised Revised January 21, 2015 January 21, 2015 This Page Intentionally Left Blank Request for Letters of Authorization for the Incidental Harassment of Marine Mammals Resulting from Navy Training Activities in the Gulf of Alaska Temporary Maritime Activities Area TABLE OF CONTENTS 1 INTRODUCTION AND DESCRIPTION OF ACTIVITIES ......................................................................1-1 1.1 INTRODUCTION ..........................................................................................................................1-1 1.2 BACKGROUND ............................................................................................................................1-3 1.3 OVERVIEW OF TRAINING ACTIVITIES ................................................................................................1-3 1.3.1 DESCRIPTION OF CURRENT TRAINING ACTIVITIES WITHIN THE STUDY AREA .................................................. 1-3 1.3.1.1 Anti-Surface Warfare .................................................................................................................. 1-4 1.3.1.2 Anti-Submarine Warfare ............................................................................................................ -

USS Midway Museum Historic Gaslamp Quarter Balboa Park
Approx. 22 Miles Approx. 28 Miles San Diego Zoo Del Mar Legoland Fairgrounds Safari Park Del Mar Beaches DOG FRIENDLY 56 North Beach 5 Torrey Pines State Natural Reserve Hiking Torrey Pines Golf Course 805 Torrey Pines Gliderport University of California San Diego Birch Aquarium at Scripps Westfield UTC Mall La Jolla Shores La Jolla Cove 52 Village of La Jolla SeaWorld USS Midway Historic Gaslamp Balboa Park & Museum Quarter San Diego Zoo Approx. 12 Miles Approx. 15 Miles Approx. 16 Miles Approx. 16 Miles Fun Things To Do Within Walking Distance Torrey Pines Golf Course (0.5 mi) – Perfect your swing at the world renowned Torrey Pines Golf Course, home to two 18-hole championship courses. This public course has a driving range and is open every day until 30 minutes before dusk. Call our Golf Team at 1-800-991-GOLF (4653) to book your tee time. Torrey Pines State Natural Reserve (0.8 mi) – Hike a trail in this beautiful 2,000-acre coastal state park overlooking the Pacific Ocean. Some trails lead directly to Torrey Pines State Beach. Trail maps available at our Concierge Desk. Torrey Pines Gliderport (1.5 mi) – Visit North America's top paragliding and hang gliding location and try an instructional tandem flight. Please call ahead since all flights are dependent on the wind conditions - (858) 452-9858. Fun Things To Do Just a Short Drive Away La Jolla Playhouse (2 mi) – A not-for-profit, professional theatre at the University of California San Diego. See Concierge for current showings. Birch Aquarium (3 mi) – Experience stunning sea life at Birch Aquarium at Scripps Institute of Oceanography. -

CELEBRATE LIKE a Legend Aloha WELCOME to DUKE’S LA JOLLA
CELEBRATE LIKE A legend Aloha WELCOME TO DUKE’S LA JOLLA Few locations in San Diego rival the oceanfront setting of Duke’s La Jolla. Our restaurant offers some of the most spectacular sweeping views of the Pacific Ocean, overlooking La Jolla Cove and La Jolla Shores. Our setting is just the beginning of what makes hosting your event at Duke’s La Jolla a truly memorable occasion. Our beautiful restaurant provides you and your guests with an authentic, nostalgic walk back in time through the evolution of surfing and the development of the surfboard. As you enter the main foyer of the restaurant each element has been specifically designed to create a mood of an older time, but in a contemporary setting. Duke’s La Jolla specializes in local sustainable fish caught daily in our local waters along with premium steaks, all natural pork, free range chicken and locally sourced produce from local farms. Every event at Duke’s is served with the warm, personalized service that is the signature of Duke’s. So whether it is a large scale brunch gathering overlooking the sparkling waters or an intimate evening reception with the backdrop of the southern California coast; Duke’s La Jolla has the perfect venue for your celebration. 2 BAREFOOT BAR LAYOUT A panoramic oceanfront setting for your event at Duke’s. This dining area on our upper level is highlighted by sweeping views of the Pacific and offers a dedicated bar opening to an ocean-view patio. The Barefoot Bar seats 30 to 120 guests and hosts up to 150 guests standing. -

Federal Register/Vol. 84, No. 111/Monday, June 10
26940 Federal Register / Vol. 84, No. 111 / Monday, June 10, 2019 / Notices DEPARTMENT OF COMMERCE and will generally be posted online at limitations indicated above and https://www.fisheries.noaa.gov/permit/ amended the definition of ‘‘harassment’’ National Oceanic and Atmospheric incidental-take-authorizations-under- as it applies to a ‘‘military readiness Administration marine-mammal-protection-act without activity.’’ The definitions of all change. All personal identifying applicable MMPA statutory terms cited RIN 0648–XG948 information (e.g., name, address) above are included in the relevant Takes of Marine Mammals Incidental to voluntarily submitted by the commenter sections below. may be publicly accessible. Do not Specified Activities; Taking Marine National Environmental Policy Act Mammals Incidental to Marine submit confidential business Geophysical Surveys in the Northeast information or otherwise sensitive or To comply with the National Pacific Ocean protected information. Environmental Policy Act of 1969 FOR FURTHER INFORMATION CONTACT: (NEPA; 42 U.S.C. 4321 et seq.) and AGENCY: National Marine Fisheries Amy Fowler, Office of Protected NOAA Administrative Order (NAO) Service (NMFS), National Oceanic and Resources, NMFS, (301) 427–8401. 216–6A, NMFS must review our Atmospheric Administration (NOAA), Electronic copies of the application and proposed action (i.e., the issuance of an Commerce. supporting documents, as well as a list incidental harassment authorization) ACTION: Notice; proposed incidental of the references cited in this document, with respect to potential impacts on the harassment authorization; request for may be obtained online at: https:// human environment. comments on proposed authorization www.fisheries.noaa.gov/permit/ Accordingly, NMFS is preparing an and possible renewal. -

San Diego County Facts and Figures
San Diego County Facts and Figures POPULATION1: EMPLOYMENT MIX: (Industry)1 Year: 2018 2019 2020 2019 2020 Employees Employees Total: 3,337,456 3,340,312 3,343,355 Government2 251,600 235,900 1San Diego County is the second most populous county in California and fifth most populous in the United States. Professional and Business Services 261,300 253,400 Source: California Department of Finance. Trade, Transportation and Utilities 232,900 220,500 Note: Population for 2019 was restated. Educational and Health Services 220,800 211,800 Leisure and Hospitality 200,600 130,400 INCORPORATED CITIES: 18 Manufacturing 117,300 112,900 Financial Activities 77,500 74,000 Construction 84,800 87,800 Other Services 54,500 40,600 CIVILIAN LABOR FORCE: Information Technology 23,500 21,900 Year: 2019 2020 Farming 9,000 8,400 Total: 1,590,600 1,538,400 Mining and Logging 400 300 Source: California Employment Development Department. Total 1,534,200 1,397,900 1Industry employment is by place of work; excludes self-employed individuals, unpaid family workers, and household domestic workers. 2Excludes the U.S. Department of Defense. Source: California Employment Development Department UNEMPLOYMENT RATE: Year: 2019 2020 TEN LARGEST EMPLOYERS: Percentage: 3.2% 9.2% 2019 2020 Source: California Employment Development Department. Employees Employees U.C. San Diego 35,847 35,802 Sharp Healthcare 18,700 19,468 County of San Diego 18,025 17,954 City of San Diego 11,545 11,820 San Diego Community College District 6,805 5,400 General Atomics (and affiliated companies) 6,777 6,745 San Diego State University 6,371 6,454 Rady Children’s Hospital-San Diego 5,541 5,711 YMCA of San Diego County 5,517 5,057 Sempra Energy 4,741 5,063 Sources: San Diego Business Journal Book of Lists (2020) & County of San Diego Fiscal Year 2019-20 Adopted Operational Plan. -

Senate Concurrent Resolution No. 17
Senate Concurrent Resolution No. 17 Introduced by Senator Block (Principal coauthor: Assembly Member Atkins) February 4, 2015 Senate Concurrent Resolution No. 17ÐRelative to Navy Reserve Day. legislative counsel’s digest SCR 17, as introduced, Block. Navy Reserve Day. This measure would commemorate March 3, 2015, as the 100th anniversary of the United States Navy Reserve. Fiscal committee: no. line 1 WHEREAS, The Naval Appropriations Act dated March 3, line 2 1915, established a United States Naval Reserve, stating ªThere line 3 is hereby established a United States Naval Reserve, which shall line 4 consist of citizens of the United States who have been or may be line 5 entitled to be honorably discharged from the Navy after not less line 6 than one four-year term of enlistment or after a term of enlistment line 7 during minority. The naval reserve shall be organized under the line 8 Bureau of Navigation and shall be governed by the Articles for line 9 the Government of the Navy and by the Naval Regulations and line 10 instructions.º; and line 11 WHEREAS, Men and women of the United States Navy Reserve line 12 have trained and served alongside their active duty counterparts line 13 in the State of California since World War I; and line 14 WHEREAS, In 1926, the Naval Reserve Of®cer Training Corps line 15 was ®rst established, entrusted to only six American universities line 16 including the University of California; and Corrected 2-17-15ÐSee last page. 99 SCR 17 Ð 2 Ð line 1 WHEREAS, Former Secretary of the Navy John L. -

RCN DIVING BRANCH HISTORY – Part 7C by Robert Larsen 2014
RCN DIVING BRANCH HISTORY – Part 7C By Robert Larsen 2014 Robert “Red” Larsen wrote that this is a continuation from Part 7B, identifying some main topics and my knowledge of how we used, developed , or used the particular subject. Diving Equipment – SCUBA or CABA(Compressed Air Breathing Apparatus) On the West Coast in 1954, we were introduced to CABA during our initial course. When I look back on it, there did not seem to be a great deal of knowledge from out Instructor’s regarding this breathing apparatus. We referred to the system as an “Aqua Lung”. The breathing regulators had a “Canada Liquid Air” nameplate on them, plus a Serial Number. I just learned via the internet why these were labelled as such – in 1947 Emile Gagnan, co‐inventor with Jacques Cousteau of the Aqua Lung, emigrated to Montreal, Quebec, and worked at Canada Liquid Air Company, developing more regulators, which were the old double hose type. They apparently were a single stage regulator, reducing the tank supply pressure from 1800/2000 PSI to the Diver’s over‐bottom pressure, in one single control stage. You probably wonder why I would remember about the serial numbers? Well, these regulators took a mighty strong set of lungs to be able to draw in your air supply – compared to our more modern equipment, and some of these were much harder than others. Also, on the surface you could identify what Serial Number the Diver was using, by the sound of the exhaust bubbles! Number “1101” was always able to be identified in this manner(Jim Balmforth and I were discussing this feature recently when I travelled out to the West Coast).