Navy Gulf of Alaska Testing and Training 2017 Rule Application
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
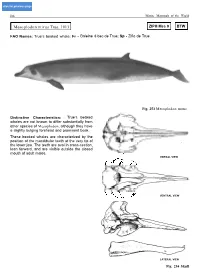
Mesoplodon Mirus True, 1913 ZIPH Mes 9 BTW
click for previous page 106 Marine Mammals of the World Mesoplodon mirus True, 1913 ZIPH Mes 9 BTW FAO Names: True’s beaked whale: Fr - Baleine à bec de True; Sp - Zifio de True. Fig. 253 Mesoplodon mirus Distinctive Characteristics: True’s beaked whales are not known to differ substantially from other species of Mesoplodon, although they have a slightly bulging forehead and prominent beak. These beaked whales are characterized by the position of the mandibular teeth at the very tip of the lower jaw. The teeth are oval in cross-section, lean forward, and are visible outside the closed mouth of adult males. DORSAL VIEW VENTRAL VIEW lLATERAL VIEWl Fig. 254 Skull Cetacea - Odontoceti - Ziphiidae 107 Can be confused with: At sea, True’s beaked whales are difficult to distinguish from other species of Mesoplodon (starting on p. 90). The only other species in which males have oval teeth at the tip of the lower jaw is Longman’s beaked whale (p. 112); whose appearance is not known. Size: Both sexes are known to reach lengths of slightly over 5 m. Weights of up to 1 400 kg have been recorded. Newborns are probably between 2 and 2.5 m. Geographical Distribution: True’s beaked whales are known only from strandings in Great Britain, from Florida to Nova Scotia in the North Atlantic, and from southeast Africa and southern Australia in the Indo-PacificcOcean. Fig. 255 Biology and Behaviour: There is almost no information available on the natural history of this species of beaked whale. Stranded animals have had squid in their stomachs. -

Federal Register/Vol. 84, No. 111/Monday, June 10
26940 Federal Register / Vol. 84, No. 111 / Monday, June 10, 2019 / Notices DEPARTMENT OF COMMERCE and will generally be posted online at limitations indicated above and https://www.fisheries.noaa.gov/permit/ amended the definition of ‘‘harassment’’ National Oceanic and Atmospheric incidental-take-authorizations-under- as it applies to a ‘‘military readiness Administration marine-mammal-protection-act without activity.’’ The definitions of all change. All personal identifying applicable MMPA statutory terms cited RIN 0648–XG948 information (e.g., name, address) above are included in the relevant Takes of Marine Mammals Incidental to voluntarily submitted by the commenter sections below. may be publicly accessible. Do not Specified Activities; Taking Marine National Environmental Policy Act Mammals Incidental to Marine submit confidential business Geophysical Surveys in the Northeast information or otherwise sensitive or To comply with the National Pacific Ocean protected information. Environmental Policy Act of 1969 FOR FURTHER INFORMATION CONTACT: (NEPA; 42 U.S.C. 4321 et seq.) and AGENCY: National Marine Fisheries Amy Fowler, Office of Protected NOAA Administrative Order (NAO) Service (NMFS), National Oceanic and Resources, NMFS, (301) 427–8401. 216–6A, NMFS must review our Atmospheric Administration (NOAA), Electronic copies of the application and proposed action (i.e., the issuance of an Commerce. supporting documents, as well as a list incidental harassment authorization) ACTION: Notice; proposed incidental of the references cited in this document, with respect to potential impacts on the harassment authorization; request for may be obtained online at: https:// human environment. comments on proposed authorization www.fisheries.noaa.gov/permit/ Accordingly, NMFS is preparing an and possible renewal. -

How to Cite Complete Issue More Information About This Article
Therya ISSN: 2007-3364 Asociación Mexicana de Mastozoología A. C. García-Grajales, Jesús; Buenrostro Silva, Alejandra; Rodríguez-Rafael, Eunice; Meraz, Juan Biological observations and first stranding record of Mesoplodon peruvianus from the central Pacific coast of Oaxaca, Mexico Therya, vol. 8, no. 2, 2017, pp. 179-184 Asociación Mexicana de Mastozoología A. C. DOI: 10.12933/therya-17-451 Available in: http://www.redalyc.org/articulo.oa?id=402350522008 How to cite Complete issue Scientific Information System Redalyc More information about this article Network of Scientific Journals from Latin America and the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Project academic non-profit, developed under the open access initiative THERYA, 2017, Vol. 8 (2): 179-184 DOI: 10.12933/therya-17-451 ISSN 2007-3364 Biological observations and first stranding record of Mesoplodon peruvianus from the central Pacific coast of Oaxaca, Mexico JESÚS GARCÍA-GRAJALES1*, ALEJANDRA BUENROSTRO SILVA2, EUNICE RODRÍGUEZ-RAFAEL3, AND JUAN MERAZ1 1 Instituto de Recursos, Universidad del Mar campus Puerto Escondido. Km 2.5, Carretera Federal Puerto Escondido – Sola de Vega, Puerto Escondido, CP. 71980, Oaxaca. Oaxaca, México. E-mail: [email protected] (JGG), [email protected] (JM) 2 Instituto de Industrias, Universidad del Mar campus Puerto Escondido. Km 2.5, Carretera Federal Puerto Escondido – Sola de Vega, Puerto Escondido, CP. 71980, Oaxaca. Oaxaca, México. E-mail: [email protected] (ABS). 3 Licenciatura en Biología Marina, Universidad del Mar. Ciudad Universitaria, Puerto Ángel, Distrito de San Pedro Pochutla, CP. 70902. Oaxaca, México. E-mail: [email protected] (ER-R). *Corresponding author The knowledge of Mesoplodon peruvianus species about its distribution and ecology is limited, because the range of the species is un- known. -

Appendix E Marine Mammal Density Report
Appendix E Marine Mammal Density Report GULF OF ALASKA NAVY TRAINING ACTIVITIES EIS/OEIS FINAL (MARCH 2011) TABLE OF CONTENTS E MARINE MAMMAL DENSITY AND DEPTH DISTRIBUTION .................................... E-1 E.1 BACKGROUND AND OVERVIEW .............................................................................................. E-1 E.1.1 DENSITY ................................................................................................................................. E-1 E.1.2 DEPTH DISTRIBUTION ......................................................................................................... E-6 E.1.3 DENSITY AND DEPTH DISTRIBUTION COMBINED ....................................................... E-6 E.2 MYSTICETES ............................................................................................................................ E-7 E.2.1 BLUE WHALE, BALAENOPTERA MUSCULUS ................................................................... E-7 E.2.2 FIN WHALE, BALAENOPTERA PHYSALUS ......................................................................... E-8 E.2.3 SEI WHALE, BALAENOPTERA BOREALIS........................................................................... E-8 E.2.4 MINKE WHALE, BALAENOPTERA ACUTOROSTRATA ..................................................... E-8 E.2.5 HUMPBACK WHALE, MEGAPTERA NOVAEANGLIAE ..................................................... E-9 E.2.6 NORTH PACIFIC RIGHT WHALE, EUBALAENA JAPONICA ............................................ E-9 E.2.7 GRAY WHALE, ESCHRICHTIUS -

Download 15 March 2014)
References of Scientific Descriptions Abel/Owen References of Scientific Descriptions Abel (1905).Abdr.Jahrb. geol. Reich.sans/. 55(2): 388 [Plwcoena jJhocoena relicta]. chus]. 43 [Steno]. 44 [Stenella allenuala allenuata]. 46 [Pontoporia]. 48 [CajJerea marginata]. pl. 34 [Cepha[{) Allen (1902). Bull. Am. Mus. Nat. Hist. 16: 485 [Phoca vilulina stejnegen]. rhynchus eutrojJia]. Anderson (1879). Anal. Znol.&s., Yunnan, 551 [BalaenojJlera edeni]. Gray (1850).Cat. Spec. Mamm.Coll. Brit. Mus. Part I. Cetacea, II5 [Stenella clymene]. Andrews (1908). Bull. Am.Mus. Nat. Hist. 24: 203 [MesojJ/odon bowdoini]. Gray (1859). Proc. Znol.Soc. London 1859: 359 [Callorhinus]. Andrews (19II). Bull. Am. Mus. Nat. Hist. 30: 31 [Phocoenoides].32 [Phocoenoides dalli truei]. Gray (1864). Ann. Mag. Nat. Hist., 3"1Ser. 14: 350 [Eschrichlius]. Baker, Smith & Pichler (2002). J !Wy.Soc. New ilal. 32: 725 [Cephalorhynchus hectori maui]. Gray (1864). Proc. Znol.Soc. London 1864: 28 [Phoca vilulina richardii], 201 [Eubalaena]. 202 [Caperea]. Barabash-Nikiforov (I 935).Bull. Soc. Nat. Moscou, Seel.Biol. 44: 246 [DeljJhinus delphis jJonticus]. Gray (1865). Proc. Znol.Soc. London 1865: 357 [MesojJlodonlayardii]. Barabash-Nikiforov (1940). Voronezhlwgo Gosudarstvennogo Univ.,,86 [TursiojJs truncatus j1onticus]. Gray (1866). Ann. Mag. Nat. Hist., 3"'Ser. 18: 231 [Neophoca]. Beasley, Robertson & Arnold (2005). Mar. Mamm. Sci. 21: 378 ['brcaella heinsohni]. Gray (1866). Cat.Seals WhalesBrit. Mus., 285 [Orcaella].401 [Sotalia]. van Bree (1971). Mammalia 35: 345 [DeljJhinus cajJensis lrojJicalis]. Gray (1866). Proc. Znol.Soc. London 1866: 213 [Sousa, Stenella]. de Blainville (1817).Nouv. Diet. Hist.Nat. 9: 151 [Inia geo]Jrensis]. 178 [Mesoplodon densiroslris]. Gray (1870).Proc. Znol.Soc. London 1870: 77 [Feresa). deBlainville (1820).J Phys. -

Section 3.7 Marine Mammals
Point Mugu Sea Range Draft EIS/OEIS April 2020 Environmental Impact Statement/ Overseas Environmental Impact Statement Point Mugu Sea Range TABLE OF CONTENTS 3.7 Marine Mammals ............................................................................................................. 3.7-1 3.7.1 Introduction .......................................................................................................... 3.7-1 3.7.2 Region of Influence ............................................................................................... 3.7-1 3.7.3 Approach to Analysis ............................................................................................ 3.7-1 3.7.4 Affected Environment ........................................................................................... 3.7-2 3.7.4.1 General Background .............................................................................. 3.7-2 3.7.4.2 Mysticete Cetaceans Expected in the Study Area ............................... 3.7-28 3.7.4.3 Odontocete Cetaceans Expected in the Study Area ............................ 3.7-50 3.7.4.4 Otariid Pinnipeds Expected in the Study Area ..................................... 3.7-79 3.7.4.5 Phocid Pinnipeds Expected in the Study Area ..................................... 3.7-85 3.7.4.6 Mustelids (Sea Otter) Expected in the Study Area .............................. 3.7-89 3.7.5 Environmental Consequences ............................................................................ 3.7-91 3.7.5.1 Long-Term Consequences .................................................................. -

Known and Inferred Distributions of Beaked Whale Species (Cetacea: Ziphiidae)
J. CETACEAN RES. MANAGE. 7(3):271–286, 2006 271 Known and inferred distributions of beaked whale species (Cetacea: Ziphiidae) COLIN D. MACLEOD*, WILLIAM F. PERRIN+, ROBERT PITMAN+, JAY BARLOW+, LISA BALLANCE+, ANGELA D’AMICO#, TIM GERRODETTE+, GERALD JOYCE**, KEITH D. MULLIN++, DEBRA L. PALKA¥, AND GORDON T. WARING¥ Contact e-mail: [email protected] ABSTRACT Information regarding beaked whales is so sparse that even the most basic aspects of their biology, such as their distribution, remain poorly defined for some species. We have reviewed the known distribution of each beaked whale species and where possible, used this information to infer its global distribution. While for some species, such as the relatively commonly recorded Cuvier’s beaked whale, the inferred distribution is likely to be an accurate reflection of the species’ actual distribution, for other lesser known species, such as the spade-toothed whale, the inferred distribution is more tentative. However, even such limited distribution information is essential when assessing and mitigating potential anthropogenic impacts on beaked whales and serves to highlight gaps in our knowledge that need to be filled if assessment and mitigation are to be successfully conducted. KEY WORDS: DISTRIBUTION; NORTHERN HEMISPHERE; SOUTHERN HEMISPHERE; CONSERVATION INTRODUCTION Stranded animals may have drifted, either incapacitated or as dead carcasses, for long distances before making landfall, To understand the extent to which beaked whales may be meaning that such evidence may not reflect the actual affected by anthropogenic activities, it is essential to know distribution of the species. Finally, while a great deal of where they occur. -

A First Confirmed Specimen Record in Chile, and Sightings Attributed to the Lesser Beaked Whale Mesoplodon Peruvianus Reyes, Mead and Van Waerebeek, 1991
BoletínH. NÚÑEZ del Museo y D. PINCHEIRA-DONOSO Nacional de Historia Natural, / Liolaemus Chile, confusus 56: 89- 96, nueva (2007) especie de lagartija de Chile central 89 A FIRST CONFIRMED SPECIMEN RECORD IN CHILE, AND SIGHTINGS attributed to THE LESSER BEAKED WHALE MESOPLODON PERUVIANUS REYES, MEAD AND VAN WAEREBEEK, 1991 G. PAOLO SANINO 1,2,3, JOSÉ L. YÁÑEZ 2,1 and KOEN VAN WAEREBEEK 1,3 1 Centre for Marine Mammals Research – Leviathan (CMMR Leviathan), PC 7640392, Santiago, Chile. e-mail: [email protected] 2 National Museum of Natural History (MNHN), Casilla 787, Santiago, Chile. e-mail: [email protected] 3 Peruvian Centre for Cetacean Research (CEPEC), Museo de Delfines, Pucusana, Peru. e-mail: [email protected] ABSTRACT Three sightings totalling five small-sized beaked whales recorded off north-central Chile (ca. 29°S) in February 1998, two between Punta Zorros and Damas Island and one south of Choros Island, were attributed to Mesoplodon peruvianus. A ca. 1m neonate was observed for the first time. The occurrence of lesser beaked whales in shallow water habitat (20-70m depth) is unusual in the Family Ziphiidae. On 17 December 1997, during the IWC 3rd Blue Whale Survey off Chile, researchers (including two of the authors) assigned another beaked whale sighting at 20º26’S, 70º44’W, in deeper water (878-1245m), as a probable M. peruvianus. All individuals shared the following characteristics: small body size, short snout, nondescript dark colouration dorsally and a low, markedly triangular dorsal fin. An adult beaked whale skull (specimen GPS004) was collected at Los Choros beach (29º17.04’S, 71º23.54’W) in May 1995. -

Section 3.4 (Marine Mammals) (U.S
3.4 Marine Mammals Mariana Islands Training and Testing Final Supplemental EIS/OEIS June 2020 Supplemental Environmental Impact Statement/ Overseas Environmental Impact Statement Mariana Islands Training and Testing TABLE OF CONTENTS 3.4 Marine Mammals ............................................................................................................. 3.4-1 3.4.1 Affected Environment........................................................................................... 3.4-1 3.4.1.1 General Background ................................................................................ 3.4-1 3.4.1.2 Species Unlikely to Be Present in the Study Area .................................... 3.4-3 3.4.1.3 Group Size ................................................................................................ 3.4-3 3.4.1.4 Habitat Use .............................................................................................. 3.4-4 3.4.1.5 Dive Behavior ........................................................................................... 3.4-4 3.4.1.6 Hearing and Vocalization ......................................................................... 3.4-4 3.4.1.7 General Threats ....................................................................................... 3.4-8 3.4.1.8 Blue Whale (Balaenoptera musculus) .................................................... 3.4-16 3.4.1.9 Bryde’s Whale (Balaenoptera edeni) ..................................................... 3.4-17 3.4.1.10 Fin Whale (Balaenoptera physalus) .................................................... -

Environmental Assessment of a Marine Geophysical Survey by the R/V Melville in the Pacific Ocean Off Central and South America, October–November 2010
Environmental Assessment of a Marine Geophysical Survey by the R/V Melville in the Pacific Ocean off Central and South America, October–November 2010 Prepared for Scripps Institution of Oceanography 8602 La Jolla Shores Drive La Jolla, CA. 92037 and National Science Foundation Division of Ocean Sciences 4201 Wilson Blvd., Suite 725 Arlington, VA 22230 by LGL Ltd., environmental research associates 22 Fisher St., POB 280 King City, Ont. L7B 1A6 25 May 2010 Revised 13 July and 16 September 2010 LGL Report TA4902-1 Table of Contents TABLE OF CONTENTS Page ABSTRACT.................................................................................................................................................. vi LIST OF ACRONYMS .................................................................................................................................. vii I. PURPOSE AND NEED ................................................................................................................................ 1 II. ALTERNATIVES INCLUDING PROPOSED ACTION .................................................................................... 2 Proposed Action ............................................................................................................................... 2 (1) Project Objectives and Context ......................................................................................... 2 (2) Proposed Activities ........................................................................................................... 2 (3) -
LDEO NE Pacific IHA Final EA 14 June 2019
Final Environmental Analysis of a Marine Geophysical Survey by R/V Marcus G. Langseth in the Northeast Pacific Ocean, Summer 2019 Prepared for Lamont-Doherty Earth Observatory 61 Route 9W, P.O. Box 1000 Palisades, NY 10964-8000 and National Science Foundation Division of Ocean Sciences 4201 Wilson Blvd., Suite 725 Arlington, VA 22230 by LGL Ltd., environmental research associates 22 Fisher St., POB 280 King City, ON L7B 1A6 13 June 2019 LGL Report FA0169-03 Table of Contents TABLE OF CONTENTS LIST OF FIGURES ..................................................................................................................................... iv LIST OF TABLES ....................................................................................................................................... v ABSTRACT .............................................................................................................................................. vi LIST OF ACRONYMS .............................................................................................................................. viii I PURPOSE AND NEED .............................................................................................................................. 1 1.1 Mission of NSF ........................................................................................................................... 1 1.2 Purpose of and Need for the Proposed Action .............................................................................. 1 1.3 Background of NSF-funded Marine -
Diapositivo 1
Index Preface ..………………………………….………………………………………. Page 3 The archipelago of the Azores ……………………………………….. Page 4 Characteristics of the Cetaceans ……………………………………. Page 5 Cetaceans classification ..……………………………………………….. Page 6 Whale hunting …………………………………………………………………. Page 7 Species ……………………………………………………………………………. Page 8 Other languages ……………………………………………………………… Page 47 The ocean and you …………………………………………………………. Page 49 Acknowledgements …………………………………………………………. Page 50 2 Preface A whale watching trip in the archipelago of the Azores, is a unique and amazing experience. It is possible to interact with cetaceans and other animals in their natural environment by seeing, getting a feeling of closeness and enjoying their behavior. It is possible to observe many different species of animals, not only cetaceans, but also birds, fish, sharks and others. The aims of this Ocean Guide are to summarize the most important species seen on these trips, giving important information and additional details that might be interesting, as well as providing representative images. It is not supposed to be a very technical guide but a tool that can Through this guide, everyone will get the chance to easily be used by any person, and consider and appreciate the unquestionable beauty that can be useful for identifying and and see how fascinating these marvelous animals are. learning about the species viewed during the trip. 3 The archipelago of Azores Located in the middle of the North Atlantic, the archipelago of the Azores is one of the best sites in Europe for the observation of Cetaceans. The nine Azorean islands are the most visible faces of a complex set of underwater mountains with over a thousand meters in depth, created by volcanic activity, due to the tectonic movements of the Eurasian plate, the African plate and the North American plate.