LDEO NE Pacific IHA Final EA 14 June 2019
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
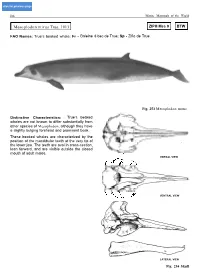
Mesoplodon Mirus True, 1913 ZIPH Mes 9 BTW
click for previous page 106 Marine Mammals of the World Mesoplodon mirus True, 1913 ZIPH Mes 9 BTW FAO Names: True’s beaked whale: Fr - Baleine à bec de True; Sp - Zifio de True. Fig. 253 Mesoplodon mirus Distinctive Characteristics: True’s beaked whales are not known to differ substantially from other species of Mesoplodon, although they have a slightly bulging forehead and prominent beak. These beaked whales are characterized by the position of the mandibular teeth at the very tip of the lower jaw. The teeth are oval in cross-section, lean forward, and are visible outside the closed mouth of adult males. DORSAL VIEW VENTRAL VIEW lLATERAL VIEWl Fig. 254 Skull Cetacea - Odontoceti - Ziphiidae 107 Can be confused with: At sea, True’s beaked whales are difficult to distinguish from other species of Mesoplodon (starting on p. 90). The only other species in which males have oval teeth at the tip of the lower jaw is Longman’s beaked whale (p. 112); whose appearance is not known. Size: Both sexes are known to reach lengths of slightly over 5 m. Weights of up to 1 400 kg have been recorded. Newborns are probably between 2 and 2.5 m. Geographical Distribution: True’s beaked whales are known only from strandings in Great Britain, from Florida to Nova Scotia in the North Atlantic, and from southeast Africa and southern Australia in the Indo-PacificcOcean. Fig. 255 Biology and Behaviour: There is almost no information available on the natural history of this species of beaked whale. Stranded animals have had squid in their stomachs. -

David Moretti Phd Thesis
ESTIMATING THE EFFECT OF MID-FREQUENCY ACTIVE SONAR ON THE POPULATION HEALTH OF BLAINVILLE'S BEAKED WHALES (MESOPLODON DENSIROSTRIS) IN THE TONGUE OF THE OCEAN David Moretti A Thesis Submitted for the Degree of PhD at the University of St Andrews 2019 Full metadata for this item is available in St Andrews Research Repository at: http://research-repository.st-andrews.ac.uk/ Please use this identifier to cite or link to this item: http://hdl.handle.net/10023/19250 This item is protected by original copyright Estimating the effect of mid-frequency active sonar on the population health of Blainville's beaked whales (Mesoplodon densirostris) in the Tongue of the Ocean David Moretti This thesis is submitted in partial fulfilment for the degree of Doctor of Philosophy (PhD) at the University of St Andrews March 2019 Abstract Passive acoustic methods were used to study the effect of mid-frequency active sonar (MFAS) on a population of Blainville’s beaked whales (Mesoplodon densirostris, Md) at the U.S. Navy Atlantic Undersea Test and Evaluation Centre (AUTEC), Bahamas. AUTEC contains an array of bottom-mounted hydrophones that can detect Md echolocation clicks. Methods to estimate abundance, the risk of behavioural disruption, and the population level effect of repeated MFAS exposure are presented. A passive acoustic abundance estimation method, a parametric equation that predicts the probability of foraging dive disruption as a function of MFAS received level and an Md bioenergetics model were developed. The effect of changes in energy flow on the demographic characteristics of an Md population were explored. Passive acoustic data from AUTEC were used to estimate the behavioural disturbance resulting from sonar operations; combined with the bioenergetic model, this suggested that the effect of sonar operations could cause an increase in a female’s age at maturity, a longer inter-calf-interval, calf survival rate and probability of giving birth that could in turn result in a declining population. -

Navy Gulf of Alaska Testing and Training 2017 Rule Application
REQUEST FOR LETTER OF AUTHORIZATION FOR THE INCIDENTAL HARASSMENT OF MARINE MAMMALS RESULTING FROM U.S. NAVY TRAINING ACTIVITIES IN THE GULF OF ALASKA TEMPORARY MARITIME ACTIVITIES AREA Submitted to: Office of Protected Resources National Marine Fisheries Service 1315 East-West Highway Silver Spring, Maryland 20910-3226 Submitted by: Commander, United States Pacific Fleet 250 Makalapa Drive Pearl Harbor, Hawaii 96860-3131 Revised Revised January 21, 2015 January 21, 2015 This Page Intentionally Left Blank Request for Letters of Authorization for the Incidental Harassment of Marine Mammals Resulting from Navy Training Activities in the Gulf of Alaska Temporary Maritime Activities Area TABLE OF CONTENTS 1 INTRODUCTION AND DESCRIPTION OF ACTIVITIES ......................................................................1-1 1.1 INTRODUCTION ..........................................................................................................................1-1 1.2 BACKGROUND ............................................................................................................................1-3 1.3 OVERVIEW OF TRAINING ACTIVITIES ................................................................................................1-3 1.3.1 DESCRIPTION OF CURRENT TRAINING ACTIVITIES WITHIN THE STUDY AREA .................................................. 1-3 1.3.1.1 Anti-Surface Warfare .................................................................................................................. 1-4 1.3.1.2 Anti-Submarine Warfare ............................................................................................................ -

Federal Register/Vol. 84, No. 111/Monday, June 10
26940 Federal Register / Vol. 84, No. 111 / Monday, June 10, 2019 / Notices DEPARTMENT OF COMMERCE and will generally be posted online at limitations indicated above and https://www.fisheries.noaa.gov/permit/ amended the definition of ‘‘harassment’’ National Oceanic and Atmospheric incidental-take-authorizations-under- as it applies to a ‘‘military readiness Administration marine-mammal-protection-act without activity.’’ The definitions of all change. All personal identifying applicable MMPA statutory terms cited RIN 0648–XG948 information (e.g., name, address) above are included in the relevant Takes of Marine Mammals Incidental to voluntarily submitted by the commenter sections below. may be publicly accessible. Do not Specified Activities; Taking Marine National Environmental Policy Act Mammals Incidental to Marine submit confidential business Geophysical Surveys in the Northeast information or otherwise sensitive or To comply with the National Pacific Ocean protected information. Environmental Policy Act of 1969 FOR FURTHER INFORMATION CONTACT: (NEPA; 42 U.S.C. 4321 et seq.) and AGENCY: National Marine Fisheries Amy Fowler, Office of Protected NOAA Administrative Order (NAO) Service (NMFS), National Oceanic and Resources, NMFS, (301) 427–8401. 216–6A, NMFS must review our Atmospheric Administration (NOAA), Electronic copies of the application and proposed action (i.e., the issuance of an Commerce. supporting documents, as well as a list incidental harassment authorization) ACTION: Notice; proposed incidental of the references cited in this document, with respect to potential impacts on the harassment authorization; request for may be obtained online at: https:// human environment. comments on proposed authorization www.fisheries.noaa.gov/permit/ Accordingly, NMFS is preparing an and possible renewal. -

Future Directions in Research on Beaked Whales
25th Meeting of the Advisory Committee ASCOBANS/AC25/Inf.5.1 Stralsund, Germany, 17-19 September 2019 Dist.16 August 2019 Agenda Item 5.1 Special Species Sessions Beaked Whales Information Document 5.1 Future Directions in Research on Beaked Whales Action Requested Take Note Submitted by Hooker et al. Note: Delegates are kindly reminded to bring their own document copies to the meeting, if needed. fmars-05-00514 January 23, 2019 Time: 17:10 # 1 REVIEW published: 25 January 2019 doi: 10.3389/fmars.2018.00514 Future Directions in Research on Beaked Whales Sascha K. Hooker1*, Natacha Aguilar De Soto2, Robin W. Baird3, Emma L. Carroll1,4, Diane Claridge1,5, Laura Feyrer6, Patrick J. O. Miller1, Aubrie Onoufriou1,2, Greg Schorr7, Eilidh Siegal1 and Hal Whitehead6 1 Sea Mammal Research Unit, Scottish Oceans Institute, University of St Andrews, St Andrews, United Kingdom, 2 BIOECOMAC Department of Animal Biology, Universidad de La Laguna, San Cristóbal de La Laguna, Spain, 3 Cascadia Research Collective, Olympia, WA, United States, 4 School of Biological Sciences, The University of Auckland, Auckland, New Zealand, 5 Bahamas Marine Mammal Research Organisation, Abaco, Bahamas, 6 Department of Biology, Dalhousie University, Halifax, NS, Canada, 7 Marine Ecology and Telemetry Research, Seabeck, WA, United States Until the 1990s, beaked whales were one of the least understood groups of large mammals. Information on northern bottlenose whales (Hyperoodon ampullatus) and Baird’s beaked whales (Berardius bairdii) was available from data collected during Edited by: Lars Bejder, whaling, however, little information existed on the smaller species other than occasional University of Hawai‘i at Manoa, data gleaned from beach-cast animals. -

Effects of Sound from Seismic Surveys on Fish Reproduction, the Management Case from Norway
Journal of Marine Science and Engineering Article Effects of Sound from Seismic Surveys on Fish Reproduction, the Management Case from Norway Lise Doksæter Sivle 1,*, Emilie Hernes Vereide 1, Karen de Jong 1 , Tonje Nesse Forland 1, John Dalen 2 and Henning Wehde 1 1 Ecosystem Acoustics Department, Institute of Marine Research, Postboks 1870 Nordnes, NO-5817 Bergen, Norway; [email protected] (E.H.V.); [email protected] (K.d.J.); [email protected] (T.N.F.); [email protected] (H.W.) 2 SoundMare, Helleveien 243, NO-5039 Bergen, Norway; [email protected] * Correspondence: [email protected] Abstract: Anthropogenic noise has been recognized as a source of concern since the beginning of the 1940s and is receiving increasingly more attention. While international focus has been on the effects of noise on marine mammals, Norway has managed seismic surveys based on the potential impact on fish stocks and fisheries since the late 1980s. Norway is, therefore, one of very few countries that took fish into account at this early stage. Until 1996, spawning grounds and spawning migration, as well as areas with drifting eggs and larvae were recommended as closed for seismic surveys. Later results showed that the effects of seismic surveys on early fish development stages were negligible at the population level, resulting in the opening of areas with drifting eggs and larvae for seismic surveys. Spawning grounds, as well as concentrated migration towards these, are still closed to seismic surveys, but the refinement of areas and periods have improved over the years. Since 2018, marine Citation: Sivle, L.D.; Vereide, E.H.; mammals have been included in the advice to management. -

How to Cite Complete Issue More Information About This Article
Therya ISSN: 2007-3364 Asociación Mexicana de Mastozoología A. C. García-Grajales, Jesús; Buenrostro Silva, Alejandra; Rodríguez-Rafael, Eunice; Meraz, Juan Biological observations and first stranding record of Mesoplodon peruvianus from the central Pacific coast of Oaxaca, Mexico Therya, vol. 8, no. 2, 2017, pp. 179-184 Asociación Mexicana de Mastozoología A. C. DOI: 10.12933/therya-17-451 Available in: http://www.redalyc.org/articulo.oa?id=402350522008 How to cite Complete issue Scientific Information System Redalyc More information about this article Network of Scientific Journals from Latin America and the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Project academic non-profit, developed under the open access initiative THERYA, 2017, Vol. 8 (2): 179-184 DOI: 10.12933/therya-17-451 ISSN 2007-3364 Biological observations and first stranding record of Mesoplodon peruvianus from the central Pacific coast of Oaxaca, Mexico JESÚS GARCÍA-GRAJALES1*, ALEJANDRA BUENROSTRO SILVA2, EUNICE RODRÍGUEZ-RAFAEL3, AND JUAN MERAZ1 1 Instituto de Recursos, Universidad del Mar campus Puerto Escondido. Km 2.5, Carretera Federal Puerto Escondido – Sola de Vega, Puerto Escondido, CP. 71980, Oaxaca. Oaxaca, México. E-mail: [email protected] (JGG), [email protected] (JM) 2 Instituto de Industrias, Universidad del Mar campus Puerto Escondido. Km 2.5, Carretera Federal Puerto Escondido – Sola de Vega, Puerto Escondido, CP. 71980, Oaxaca. Oaxaca, México. E-mail: [email protected] (ABS). 3 Licenciatura en Biología Marina, Universidad del Mar. Ciudad Universitaria, Puerto Ángel, Distrito de San Pedro Pochutla, CP. 70902. Oaxaca, México. E-mail: [email protected] (ER-R). *Corresponding author The knowledge of Mesoplodon peruvianus species about its distribution and ecology is limited, because the range of the species is un- known. -

Behavioral Responses of Beaked Whales and Other Cetaceans to Controlled Exposures of Sildimulated Sonar and Other Sounds Brandon L
Behavioral responses of beaked whales and other cetaceans to controlled exposures of sildimulated sonar and other sounds Brandon L. Southall*1,2, Peter L. Tyack3, David Moretti4, Chr is top her Clar k5, Diane Clar idge6, and Ian BdBoyd7 18th Biennial Conference on the Biology of Marine Mammals Quebec City, Quebec, Canada 12‐16 October 2009 (1) Southall Environmental Associates, Inc., Santa Cruz, CA , USA (2) University of California, Santa Cruz, Long Marine Laboratory, Santa Cruz, CA, USA (3) Woods Hole Oceanographic Institution, Woods Hole, MA , USA (4) Naval Undersea Warfare Center Division, Code 71, Newport, RI, USA (5) Bioacoustics Research Program, Cornell University, Ithaca NY, USA (6) Bahamas Marine Mammal Research Organization, Sandy Point, Abaco, Bahamas (7) Sea Mammal Research Unit, University of St. Andrews, Scotland, UK Photo taken under U.S. NMFS permit # 1121‐1900 * Former Affiliation: National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Office of Science and Technology, Ocean Acoustics Program, Silver Spring, Maryland 20910‐6233, USA. Behavioral Response Study 2007‐08: Spponsors and Particippgating Organizations Atlantic Undersea Testing and Evaluation Center: Jose Arteiro; Marc Cimonella; Tod Michaelis Bahamas Marine Mammal Research Organization: Edward Adderley; Monica Arso; Diane Claridge; Charlotte Dunn; Kuame Finlayson; Leigh Hickmott; Alesha Naranjit; Olivia Patterson Cornell University: David Brown; Christopher Clark; Ian Fein Duke University: Ari Friedlaender; Douglas Nowacek; Elliot -

Appendix E Marine Mammal Density Report
Appendix E Marine Mammal Density Report GULF OF ALASKA NAVY TRAINING ACTIVITIES EIS/OEIS FINAL (MARCH 2011) TABLE OF CONTENTS E MARINE MAMMAL DENSITY AND DEPTH DISTRIBUTION .................................... E-1 E.1 BACKGROUND AND OVERVIEW .............................................................................................. E-1 E.1.1 DENSITY ................................................................................................................................. E-1 E.1.2 DEPTH DISTRIBUTION ......................................................................................................... E-6 E.1.3 DENSITY AND DEPTH DISTRIBUTION COMBINED ....................................................... E-6 E.2 MYSTICETES ............................................................................................................................ E-7 E.2.1 BLUE WHALE, BALAENOPTERA MUSCULUS ................................................................... E-7 E.2.2 FIN WHALE, BALAENOPTERA PHYSALUS ......................................................................... E-8 E.2.3 SEI WHALE, BALAENOPTERA BOREALIS........................................................................... E-8 E.2.4 MINKE WHALE, BALAENOPTERA ACUTOROSTRATA ..................................................... E-8 E.2.5 HUMPBACK WHALE, MEGAPTERA NOVAEANGLIAE ..................................................... E-9 E.2.6 NORTH PACIFIC RIGHT WHALE, EUBALAENA JAPONICA ............................................ E-9 E.2.7 GRAY WHALE, ESCHRICHTIUS -

Geophysical Surveys in the Arctic Ocean
Geophysical Surveys by R/V Sikuliaq in the Arctic Ocean #25662 National Science Foundation References Cited Aars, J., N.J. Lunn, and A.E. Derocher (eds.) 2006. Polar bears: proceedings of the 14th working meeting of the IUCN/SSC Polar Bear Specialist Group, 20–24 June, Seattle, WA, USA. IUCN, Gland, Switzerland. 189 p. Aarts, G., A.M. von Benda-Beckmann, K. Lucke, H.Ö. Sertlek, R. Van Bemmelen, S.C. Geelhoed, S. Brasseur, M. Scheidat, F.P.A. Lam, H. Slabbekoorn, and R. Kirkwood. 2016. Harbour porpoise movement strategy affects cumulative number of animals acoustically exposed to underwater explosions. Mar. Ecol. Prog. Ser. 557:261-275. Acosta, A., N. Nino-Rodriquez, M.C. Yepes, and O. Boisseau. 2017. Mitigation provisions to be implemented for marine seismic surveying in Latin America: a review based on fish and cetaceans. Aquat. Biol. 199-216. Aerts, L.A., A.E. McFarland, B.H. Watts, K.S. Lomac-MacNair, P.E. Seiser, S.S. Wisdom, A.V. Kirk, and C.A. Schudel. 2013. Marine mammal distribution and abundance in an offshore sub-region of the northeastern Chukchi Sea during the open-water season. Cont. Shelf Res. 67:116-126. Aguilar A. and R. García-Vernet. 2018. Fin whale Balaenoptera physalus. p. 368-371 In: B. Würsig, J.G.M. Thewissen, and K.M. Kovacs (eds.), Encyclopedia of Marine Mammals, 3rd ed. Academic Press/Elsevier, San Diego, CA. 1157 p. Aguilar Soto, N., M. Johnson, P.T. Madsen, P.L. Tyack, A. Bocconcelli, and J.F. Borsani. 2006. Does intense ship noise disrupt foraging in deep-diving Cuvier’s beaked whales (Ziphius cavirostris)? Mar. -
![Pageflex Server [Document: D-Aalto-70CCD9D4 00001]](https://docslib.b-cdn.net/cover/8920/pageflex-server-document-d-aalto-70ccd9d4-00001-2638920.webp)
Pageflex Server [Document: D-Aalto-70CCD9D4 00001]
Department of Signal Processing and Acoustics Aalto- Figure on the front cover is the spectrogram AriPoikonen of a sound signal measured on the bottom of DD Measurements, the sea while a broadband underwater sound 18 / source passes the hydrophone. The 2012 interference pattern in the figure is the analysis and Lloyd's mirror effect, which arises from analysiswater modelingand wind-drivenbrackish Measurements, ofambientnoiseshallow in constructive and destructive interference modeling of wind- between direct and surface-reflected sound waves. driven ambient noise in shallow brackish water Ari Poikonen 9HSTFMG*aefbdj+ 9HSTFMG*aefbdj+ ISBN 978-952-60-4513-9 BUSINESS + ISBN 978-952-60-4514-6 (pdf) ECONOMY ISSN-L 1799-4934 ISSN 1799-4934 ART + ISSN 1799-4942 (pdf) DESIGN + ARCHITECTURE UniversityAalto Aalto University School of Electrical Engineering SCIENCE + Department of Signal Processing and Acoustics TECHNOLOGY www.aalto.fi CROSSOVER DOCTORAL DOCTORAL DISSERTATIONS DISSERTATIONS Aalto University publication series DOCTORAL DISSERTATIONS 18/2012 Measurements, analysis and modeling of wind-driven ambient noise in shallow brackish water Ari Poikonen Doctoral dissertation for the degree of Doctor of Science in Technology to be presented with due permission of the School of Electrical Engineering for public examination and debate in Auditorium S1 at the Aalto University School of Electrical Engineering (Espoo, Finland) on the 9th of March 2012 at 12 noon. Aalto University School of Electrical Engineering Department of Signal Processing and Acoustics Supervisor Prof. Unto K. Laine, Aalto University, Finland Instructor Dr. Martti Kalliomäki (ret.), Finnish Naval Research Institute, Finland Preliminary examiners Prof. Pekka Heikkinen, University of Helsinki, Finland Dr. Seppo Uosukainen, Technical Research Centre of Finland (VTT), Finland Opponent Dr. -

Download 15 March 2014)
References of Scientific Descriptions Abel/Owen References of Scientific Descriptions Abel (1905).Abdr.Jahrb. geol. Reich.sans/. 55(2): 388 [Plwcoena jJhocoena relicta]. chus]. 43 [Steno]. 44 [Stenella allenuala allenuata]. 46 [Pontoporia]. 48 [CajJerea marginata]. pl. 34 [Cepha[{) Allen (1902). Bull. Am. Mus. Nat. Hist. 16: 485 [Phoca vilulina stejnegen]. rhynchus eutrojJia]. Anderson (1879). Anal. Znol.&s., Yunnan, 551 [BalaenojJlera edeni]. Gray (1850).Cat. Spec. Mamm.Coll. Brit. Mus. Part I. Cetacea, II5 [Stenella clymene]. Andrews (1908). Bull. Am.Mus. Nat. Hist. 24: 203 [MesojJ/odon bowdoini]. Gray (1859). Proc. Znol.Soc. London 1859: 359 [Callorhinus]. Andrews (19II). Bull. Am. Mus. Nat. Hist. 30: 31 [Phocoenoides].32 [Phocoenoides dalli truei]. Gray (1864). Ann. Mag. Nat. Hist., 3"1Ser. 14: 350 [Eschrichlius]. Baker, Smith & Pichler (2002). J !Wy.Soc. New ilal. 32: 725 [Cephalorhynchus hectori maui]. Gray (1864). Proc. Znol.Soc. London 1864: 28 [Phoca vilulina richardii], 201 [Eubalaena]. 202 [Caperea]. Barabash-Nikiforov (I 935).Bull. Soc. Nat. Moscou, Seel.Biol. 44: 246 [DeljJhinus delphis jJonticus]. Gray (1865). Proc. Znol.Soc. London 1865: 357 [MesojJlodonlayardii]. Barabash-Nikiforov (1940). Voronezhlwgo Gosudarstvennogo Univ.,,86 [TursiojJs truncatus j1onticus]. Gray (1866). Ann. Mag. Nat. Hist., 3"'Ser. 18: 231 [Neophoca]. Beasley, Robertson & Arnold (2005). Mar. Mamm. Sci. 21: 378 ['brcaella heinsohni]. Gray (1866). Cat.Seals WhalesBrit. Mus., 285 [Orcaella].401 [Sotalia]. van Bree (1971). Mammalia 35: 345 [DeljJhinus cajJensis lrojJicalis]. Gray (1866). Proc. Znol.Soc. London 1866: 213 [Sousa, Stenella]. de Blainville (1817).Nouv. Diet. Hist.Nat. 9: 151 [Inia geo]Jrensis]. 178 [Mesoplodon densiroslris]. Gray (1870).Proc. Znol.Soc. London 1870: 77 [Feresa). deBlainville (1820).J Phys.