Habitat-Based Spatial Models of Cetacean Density in the Eastern Pacific Ocean
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Morphology and Distribution of the Spinner Dolphin, Stenella
J. CETACEAN RES. MANAGE. 1(2):167- 177 , 1999 167 Morphology and distribution of the spinner dolphin,Stenella longirostris, rough-toothed dolphin,Steno bredanensis and melon-headed whale,Peponocephala electra, from waters off the Sultanate of Oman1 Koen Van W aerebeek*, M ichael G allagheri Robert Baldwin Vassili Papastavrou§ and Samira M ustafa A l - L a w a t i 1 Contact e-mail: [email protected] ABSTRACT The morphology of three tropical delphinids from the Sultanate of Oman and their occurrence in the Arabian Sea are presented. Body lengths of four physically mature spinner dolphins (three males) ranged from 154-178.3cm (median 164.5cm), i.e. smaller than any known stock of spinner dolphins, except the dwarf forms from Thailand and Australia. Skulls of Oman spinner dolphins (n = 10) were practically indistinguishable from those of eastern spinner dolphins ( Stenella longirostris orientalis) from the eastern tropical Pacific, but were considerably smaller than skulls of populations of pantropical ( Stenella longirostris longirostris) and Central American spinner dolphins (Stenella longirostris centroamericana). Two colour morphs (CM) were observed. The most common (CM1) has the typical tripartite pattem of the pantropical spinner dolphin. A small morph (CM2), so far seen mostly off Muscat, is characterised by a dark dorsal overlay obscuring most of the tripartite pattern and by a pinkish or white ventral field and supragenital patch. Two skulls were linked to a CM1 colour morph, the others were undetermined. It is concluded that Oman spinner dolphins should be treated as a discrete population, morphologically distinct from all known spinner dolphin subspecies. -
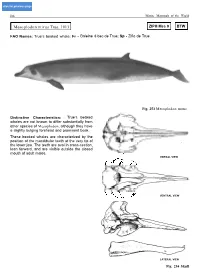
Mesoplodon Mirus True, 1913 ZIPH Mes 9 BTW
click for previous page 106 Marine Mammals of the World Mesoplodon mirus True, 1913 ZIPH Mes 9 BTW FAO Names: True’s beaked whale: Fr - Baleine à bec de True; Sp - Zifio de True. Fig. 253 Mesoplodon mirus Distinctive Characteristics: True’s beaked whales are not known to differ substantially from other species of Mesoplodon, although they have a slightly bulging forehead and prominent beak. These beaked whales are characterized by the position of the mandibular teeth at the very tip of the lower jaw. The teeth are oval in cross-section, lean forward, and are visible outside the closed mouth of adult males. DORSAL VIEW VENTRAL VIEW lLATERAL VIEWl Fig. 254 Skull Cetacea - Odontoceti - Ziphiidae 107 Can be confused with: At sea, True’s beaked whales are difficult to distinguish from other species of Mesoplodon (starting on p. 90). The only other species in which males have oval teeth at the tip of the lower jaw is Longman’s beaked whale (p. 112); whose appearance is not known. Size: Both sexes are known to reach lengths of slightly over 5 m. Weights of up to 1 400 kg have been recorded. Newborns are probably between 2 and 2.5 m. Geographical Distribution: True’s beaked whales are known only from strandings in Great Britain, from Florida to Nova Scotia in the North Atlantic, and from southeast Africa and southern Australia in the Indo-PacificcOcean. Fig. 255 Biology and Behaviour: There is almost no information available on the natural history of this species of beaked whale. Stranded animals have had squid in their stomachs. -

213 Subpart I—Taking and Importing Marine Mammals
National Marine Fisheries Service/NOAA, Commerce Pt. 218 regulations or that result in no more PART 218—REGULATIONS GOV- than a minor change in the total esti- ERNING THE TAKING AND IM- mated number of takes (or distribution PORTING OF MARINE MAM- by species or years), NMFS may pub- lish a notice of proposed LOA in the MALS FEDERAL REGISTER, including the asso- ciated analysis of the change, and so- Subparts A–B [Reserved] licit public comment before issuing the Subpart C—Taking Marine Mammals Inci- LOA. dental to U.S. Navy Marine Structure (c) A LOA issued under § 216.106 of Maintenance and Pile Replacement in this chapter and § 217.256 for the activ- Washington ity identified in § 217.250 may be modi- fied by NMFS under the following cir- 218.20 Specified activity and specified geo- cumstances: graphical region. (1) Adaptive Management—NMFS 218.21 Effective dates. may modify (including augment) the 218.22 Permissible methods of taking. existing mitigation, monitoring, or re- 218.23 Prohibitions. porting measures (after consulting 218.24 Mitigation requirements. with Navy regarding the practicability 218.25 Requirements for monitoring and re- porting. of the modifications) if doing so cre- 218.26 Letters of Authorization. ates a reasonable likelihood of more ef- 218.27 Renewals and modifications of Let- fectively accomplishing the goals of ters of Authorization. the mitigation and monitoring set 218.28–218.29 [Reserved] forth in the preamble for these regula- tions. Subpart D—Taking Marine Mammals Inci- (i) Possible sources of data that could dental to U.S. Navy Construction Ac- contribute to the decision to modify tivities at Naval Weapons Station Seal the mitigation, monitoring, or report- Beach, California ing measures in a LOA: (A) Results from Navy’s monitoring 218.30 Specified activity and specified geo- graphical region. -

FC Inshore Cetacean Species Identification
Falklands Conservation PO BOX 26, Falkland Islands, FIQQ 1ZZ +500 22247 [email protected] www.falklandsconservation.com FC Inshore Cetacean Species Identification Introduction This guide outlines the key features that can be used to distinguish between the six most common cetacean species that inhabit Falklands' waters. A number of additional cetacean species may occasionally be seen in coastal waters, for example the fin whale (Balaenoptera physalus), the humpback whale (Megaptera novaeangliae), the long-finned pilot whale (Globicephala melas) and the dusky dolphin (Lagenorhynchus obscurus). A full list of the species that have been documented to date around the Falklands can be found in Appendix 1. Note that many of these are typical of deeper, oceanic waters, and are unlikely to be encountered along the coast. The six species (or seven species, including two species of minke whale) described in this document are observed regularly in shallow, nearshore waters, and are the focus of this identification guide. Questions and further information For any questions about species identification then please contact the Cetaceans Project Officer Caroline Weir who will be happy to help you try and identify your sighting: Tel: 22247 Email: [email protected] Useful identification guides If you wish to learn more about the identification features of various species, some comprehensive field guides (which include all cetacean species globally) include: Handbook of Whales, Dolphins and Porpoises by Mark Carwardine. 2019. Marine Mammals of the World: A Comprehensive Guide to Their Identification by Thomas A. Jefferson, Marc A. Webber, and Robert L. Pitman. 2015. Whales, Dolphins and Seals: A Field Guide to the Marine Mammals of the World by Hadoram Shirihai and Brett Jarrett. -

Fall12 Rare Southern California Sperm Whale Sighting
Rare Southern California Sperm Whale Sighting Dolphin/Whale Interaction Is Unique IN MAY 2011, a rare occurrence The sperm whale sighting off San of 67 minutes as the whales traveled took place off the Southern California Diego was exciting not only because slowly east and out over the edge of coast. For the first time since U.S. of its rarity, but because there were the underwater ridge. The adult Navy-funded aerial surveys began in also two species of dolphins, sperm whales undertook two long the area in 2008, a group of 20 sperm northern right whale dolphins and dives lasting about 20 minutes each; whales, including four calves, was Risso’s dolphins, interacting with the the calves surfaced earlier, usually in seen—approximately 24 nautical sperm whales in a remarkable the company of one adult whale. miles west of San Diego. manner. To the knowledge of the During these dives, the dolphins researchers who conducted this aerial remained at the surface and Operating under a National Marine survey, this type of inter-species asso- appeared to wait for the sperm Fisheries Service (NMFS) permit, the ciation has not been previously whales to re-surface. U.S. Navy has been conducting aerial reported. Video and photographs surveys of marine mammal and sea Several minutes after the sperm were taken of the group over a period turtle behavior in the near shore and whales were first seen, the Risso’s offshore waters within the Southern California Range Complex (SOCAL) since 2008. During a routine survey the morning of 14 May 2011, the sperm whales were sighted on the edge of an offshore bank near a steep drop-off. -

Navy Gulf of Alaska Testing and Training 2017 Rule Application
REQUEST FOR LETTER OF AUTHORIZATION FOR THE INCIDENTAL HARASSMENT OF MARINE MAMMALS RESULTING FROM U.S. NAVY TRAINING ACTIVITIES IN THE GULF OF ALASKA TEMPORARY MARITIME ACTIVITIES AREA Submitted to: Office of Protected Resources National Marine Fisheries Service 1315 East-West Highway Silver Spring, Maryland 20910-3226 Submitted by: Commander, United States Pacific Fleet 250 Makalapa Drive Pearl Harbor, Hawaii 96860-3131 Revised Revised January 21, 2015 January 21, 2015 This Page Intentionally Left Blank Request for Letters of Authorization for the Incidental Harassment of Marine Mammals Resulting from Navy Training Activities in the Gulf of Alaska Temporary Maritime Activities Area TABLE OF CONTENTS 1 INTRODUCTION AND DESCRIPTION OF ACTIVITIES ......................................................................1-1 1.1 INTRODUCTION ..........................................................................................................................1-1 1.2 BACKGROUND ............................................................................................................................1-3 1.3 OVERVIEW OF TRAINING ACTIVITIES ................................................................................................1-3 1.3.1 DESCRIPTION OF CURRENT TRAINING ACTIVITIES WITHIN THE STUDY AREA .................................................. 1-3 1.3.1.1 Anti-Surface Warfare .................................................................................................................. 1-4 1.3.1.2 Anti-Submarine Warfare ............................................................................................................ -

Federal Register/Vol. 84, No. 111/Monday, June 10
26940 Federal Register / Vol. 84, No. 111 / Monday, June 10, 2019 / Notices DEPARTMENT OF COMMERCE and will generally be posted online at limitations indicated above and https://www.fisheries.noaa.gov/permit/ amended the definition of ‘‘harassment’’ National Oceanic and Atmospheric incidental-take-authorizations-under- as it applies to a ‘‘military readiness Administration marine-mammal-protection-act without activity.’’ The definitions of all change. All personal identifying applicable MMPA statutory terms cited RIN 0648–XG948 information (e.g., name, address) above are included in the relevant Takes of Marine Mammals Incidental to voluntarily submitted by the commenter sections below. may be publicly accessible. Do not Specified Activities; Taking Marine National Environmental Policy Act Mammals Incidental to Marine submit confidential business Geophysical Surveys in the Northeast information or otherwise sensitive or To comply with the National Pacific Ocean protected information. Environmental Policy Act of 1969 FOR FURTHER INFORMATION CONTACT: (NEPA; 42 U.S.C. 4321 et seq.) and AGENCY: National Marine Fisheries Amy Fowler, Office of Protected NOAA Administrative Order (NAO) Service (NMFS), National Oceanic and Resources, NMFS, (301) 427–8401. 216–6A, NMFS must review our Atmospheric Administration (NOAA), Electronic copies of the application and proposed action (i.e., the issuance of an Commerce. supporting documents, as well as a list incidental harassment authorization) ACTION: Notice; proposed incidental of the references cited in this document, with respect to potential impacts on the harassment authorization; request for may be obtained online at: https:// human environment. comments on proposed authorization www.fisheries.noaa.gov/permit/ Accordingly, NMFS is preparing an and possible renewal. -

Convention on Migratory Species
Distr: General CONVENTION ON CMS/PIC/MoS3/Inf.3.1.4 MIGRATORY 6 September 2012 SPECIES Original: English THIRD MEETING OF THE SIGNATORIES TO THE MEMORANDUM OF UNDERSTANDING FOR THE CONSERVATION OF CETACEANS AND THEIR HABITATS IN THE PACIFIC ISLANDS REGION Noumea, New Caledonia, 8 September 2012 Agenda Item 3.1 BUILDING ON THE LOCAL KNOWLEDGE OF WHALES AND DOLPHINS ALONG THE SOUTHERN COAST OF UPOLU AND THE NORTHWESTERN COAST OF SAVAI’I For reasons of economy, this document is printed in a limited number, and will not be distributed at the meeting. Delegates are kindly requested to bring their copy to the meeting and not to request additional copies. BUILDING ON THE LOCAL KNOWLEDGE OF WHALES AND DOLPHINS ALONG THE SOUTHERN COAST OF UPOLU AND THE NORTHWESTERN COAST OF SAVAI’I 20TH SEPTEMBER – 29TH OCTOBER 2010 Prepared by: Juney Ward, Malama Momoemausu, Pulea Ifopo, Titimanu Simi, Ieru Solomona1 1. Division of Environment & Conservation Staff, Ministry of Natural Resources & Environment TABLE OF CONTENTS 1. INTRODUTION ..................................................................................... 2 2. SURVEY OBJECTIVES .......................................................................... 3 3. METHODOLOGY ................................................................................ 3 - 4 a. Study area ................................................................................ 3 b. Data collection ........................................................................ 4 c. Photo-identification ................................................................. -

ARTICLE in PRESS Aquatic Mammals 2019, 45(3), Xxx-Xxx, DOI 10.1578/AM.45.3.2019.Xxx
ARTICLE IN PRESS Aquatic Mammals 2019, 45(3), xxx-xxx, DOI 10.1578/AM.45.3.2019.xxx Incidence of Odontocetes with Dorsal Fin Collapse in Maui Nui, Hawaii Stephanie H. Stack, Jens J. Currie, Jessica A. McCordic, and Grace L. Olson Pacific Whale Foundation, 300 Ma‘alaea Road, Suite 211, Wailuku, HI 96793, USA E-mail: [email protected] Abstract Introduction We examined the incidence of bent or col- A variety of dorsal fin injuries have been docu- lapsed dorsal fins of eight species of odontocetes mented in several odontocete species worldwide, observed in the nearshore waters of Maui Nui, but laterally bent or collapsed dorsal fins are a rel- Hawaii. Between 1995 and 2017, 1,312 distinc- atively uncommon occurrence (Baird & Gorgone, tive individual odontocetes were photographically 2005; Van Waerebeek et al., 2007; Luksenburg, documented. Our photo-identification catalogs in- 2014). Dorsal fin collapse is rare in wild popu- clude 583 spinner dolphins (Stenella longirostris lations of odontocetes, with published rates longirostris), 164 bottlenose dolphins (Tursiops generally less than 1%, if at all present (Baird truncatus), 132 short-finned pilot whales (Glo- & Gorgone, 2005). Exceptions to this are well- bicephala macrorhynchus), 253 pantropical studied populations of killer whales (Orcinus spotted dolphins (Stenella attenuata), 82 false orca) and the main Hawaiian Islands’ popula- killer whales (Pseudorca crassidens), 70 melon- tion of false killer whales (Pseudorca crassidens) headed whales (Peponocephala electra), 15 (Visser, 1998; Alves et al., 2017). A recent pub- pygmy killer whales (Feresa attenuata), and 13 lication by Alves et al. (2017) documented 17 rough-toothed dolphins (Steno bredanensis). -

Nai'a Or Spinner Dolphin
Courtesy Cascadia Research. Photo taken under MMPA Scientific Research Permit No. 731 © Dan McSweeney Marine Mammals Nai‘a or Spinner dolphin Stenella longirostris SPECIES STATUS: IUCN Red List - Lower Risk/ Conservation Dependent SPECIES INFORMATION: Nai‘a or spinner dolphins (Stenella longirostris) congregate into large groups and swim offshore to depths of 200 to 300 meters (650 to 1,000 feet) to feed on mesopelagic prey that includes squid, fish and shrimp. Although in large groups, they also feed in cooperative pairs or groups of pairs offshore. This foraging begins in late afternoon and continues throughout the night as the “deep scattering layer” moves closer to the surface. Recent research shows that this food source is close to shore early in the night so the spinner dolphins follow them inshore for awhile. During the day, they expend less energy resting or socializing in nearshore, shallow waters such as bays and lagoons surrounded by reef. They also may stay in their nearshore habitat to avoid predators such as sharks and killer whales. The change in group size from daytime activities to nighttime feeding is unique to Hawaii’s spinner dolphins. Although spinner dolphins are able to give birth at any time during the year, they typically show one or more seasonal peaks. Multiple males may mate with one female in short, consecutive intervals. Gestation lasts approximately ten and a half months and lactation occurs for one to two years. The calving interval is approximately three years. Additionally, the spinner dolphin is very notable for its ability to leap high out of the water while also spinning multiple times on its longitudinal axis. -

Marine Mammal Taxonomy
Marine Mammal Taxonomy Kingdom: Animalia (Animals) Phylum: Chordata (Animals with notochords) Subphylum: Vertebrata (Vertebrates) Class: Mammalia (Mammals) Order: Cetacea (Cetaceans) Suborder: Mysticeti (Baleen Whales) Family: Balaenidae (Right Whales) Balaena mysticetus Bowhead whale Eubalaena australis Southern right whale Eubalaena glacialis North Atlantic right whale Eubalaena japonica North Pacific right whale Family: Neobalaenidae (Pygmy Right Whale) Caperea marginata Pygmy right whale Family: Eschrichtiidae (Grey Whale) Eschrichtius robustus Grey whale Family: Balaenopteridae (Rorquals) Balaenoptera acutorostrata Minke whale Balaenoptera bonaerensis Arctic Minke whale Balaenoptera borealis Sei whale Balaenoptera edeni Byrde’s whale Balaenoptera musculus Blue whale Balaenoptera physalus Fin whale Megaptera novaeangliae Humpback whale Order: Cetacea (Cetaceans) Suborder: Odontoceti (Toothed Whales) Family: Physeteridae (Sperm Whale) Physeter macrocephalus Sperm whale Family: Kogiidae (Pygmy and Dwarf Sperm Whales) Kogia breviceps Pygmy sperm whale Kogia sima Dwarf sperm whale DOLPHIN R ESEARCH C ENTER , 58901 Overseas Hwy, Grassy Key, FL 33050 (305) 289 -1121 www.dolphins.org Family: Platanistidae (South Asian River Dolphin) Platanista gangetica gangetica South Asian river dolphin (also known as Ganges and Indus river dolphins) Family: Iniidae (Amazon River Dolphin) Inia geoffrensis Amazon river dolphin (boto) Family: Lipotidae (Chinese River Dolphin) Lipotes vexillifer Chinese river dolphin (baiji) Family: Pontoporiidae (Franciscana) -

Review of Small Cetaceans. Distribution, Behaviour, Migration and Threats
Review of Small Cetaceans Distribution, Behaviour, Migration and Threats by Boris M. Culik Illustrations by Maurizio Wurtz, Artescienza Marine Mammal Action Plan / Regional Seas Reports and Studies no. 177 Published by United Nations Environment Programme (UNEP) and the Secretariat of the Convention on the Conservation of Migratory Species of Wild Animals (CMS). Review of Small Cetaceans. Distribution, Behaviour, Migration and Threats. 2004. Compiled for CMS by Boris M. Culik. Illustrations by Maurizio Wurtz, Artescienza. UNEP / CMS Secretariat, Bonn, Germany. 343 pages. Marine Mammal Action Plan / Regional Seas Reports and Studies no. 177 Produced by CMS Secretariat, Bonn, Germany in collaboration with UNEP Coordination team Marco Barbieri, Veronika Lenarz, Laura Meszaros, Hanneke Van Lavieren Editing Rüdiger Strempel Design Karina Waedt The author Boris M. Culik is associate Professor The drawings stem from Prof. Maurizio of Marine Zoology at the Leibnitz Institute of Wurtz, Dept. of Biology at Genova Univer- Marine Sciences at Kiel University (IFM-GEOMAR) sity and illustrator/artist at Artescienza. and works free-lance as a marine biologist. Contact address: Contact address: Prof. Dr. Boris Culik Prof. Maurizio Wurtz F3: Forschung / Fakten / Fantasie Dept. of Biology, Genova University Am Reff 1 Viale Benedetto XV, 5 24226 Heikendorf, Germany 16132 Genova, Italy Email: [email protected] Email: [email protected] www.fh3.de www.artescienza.org © 2004 United Nations Environment Programme (UNEP) / Convention on Migratory Species (CMS). This publication may be reproduced in whole or in part and in any form for educational or non-profit purposes without special permission from the copyright holder, provided acknowledgement of the source is made.