Miissbauer Spectral Study of Ferruginous One-Layer Trioctahedral
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical and Structural Evolution of “Metamorphic Vermiculite” in Metaclastic Rocks of the Betic Cordillera, Málaga, Spain: a Synthesis
249 The Canadian Mineralogist Vol. 44, pp. 249-265 (2006) CHEMICAL AND STRUCTURAL EVOLUTION OF “METAMORPHIC VERMICULITE” IN METACLASTIC ROCKS OF THE BETIC CORDILLERA, MÁLAGA, SPAIN: A SYNTHESIS MARÍA DOLORES RUIZ CRUZ§ Departamento de Química Inorgánica, Cristalografía y Mineralogía, Universidad de Málaga, Campus de Teatinos, E-29071 Málaga, Spain JOSÉ MIGUEL NIETO Departamento de Geología, Facultad de Ciencias Experimentales, Universidad de Huelva, E-21071 Huelva, Spain ABSTRACT Vermiculite-like minerals from a metamorphic sequence of the Betic Cordillera, near Málaga, southeastern Spain, were investigated in detail by X-ray diffraction, electron-microprobe analysis, and transmission-analytical electron microscopy. Our results reveal that the chlorite-to-biotite transformation is much more complex than previously assumed. In addition to mixed- layer minerals with a mica:chlorite ratio of 2:1 and 1:1, which had previously been identifi ed, mixed-layer phases with very high and very low chlorite:vermiculite ratios have been identifi ed, together with true vermiculite, as intermediate steps in the chlorite-to-biotite transformation. The observed sequence is: chlorite → random to ordered 1:2 mica–chlorite mixed-layer phases → regular 1:1 chlorite–vermiculite mixed-layer phases → Vrm-rich chlorite–vermiculite mixed-layer phases → vermiculite → biotite. This sequence includes a continuous increase of interlayer cation content, and is similar to that described in the smectite- to-illite transformation. The high Na content of most of these phases suggests that in the absence of K-feldspar, two parallel sites of reactants, chlorite + phengite and chlorite + albite, account for the formation of vermiculitic phases, and later, of biotite. Keywords: mixed-layer minerals, metamorphic vermiculite, X-ray diffraction, electron-microprobe analysis, transmission elec- tron microscopy, analytical electron microscopy, Betic Cordillera, Spain. -

Muscovite Solid Solutions in the System K20-Mgo-Feo-A1203-Sio2-H20: an Experimental Study at 2 Kbar Ph2o and Comparison with Natural Li-Free White Micas
MINERALOGICAL MAGAZINE, JUNE 1986, VOL. 50, PP. 257-66 Muscovite solid solutions in the system K20-MgO-FeO-A1203-SiO2-H20: an experimental study at 2 kbar PH2o and comparison with natural Li-free white micas GILLES MoNIER Laboratoire de P&rologie, Universit6 d'Or16ans, 45046 Orlrans Cedex, France AND JI~AN-LouIs ROBERT Centre de Recherche sur la Synthrse et Chimie des MinSraux, G.I.S.C.N.R.S.-B.R.G.M., 1A rue de la Frrollerie, 45071 Odrans Cedex 2, France ABSTRACT. This paper presents the results of an experi- K EY W OR O S : muscovite, phengite, solid solution, crystal- mental study of muscovite solid solutions in the system chemistry, experimental mineralogy, granites, hydro- K20-M~+O-A1203 SiO2-H20 (HF), with M2+= thermal alteration. Mg 2+ or Fe 2§ in the temperature range 300 700~ under 2 kbar P.~o, Muscovite solid solutions can be described, in this system, as the result of two substitutions. NATURAL lithium-free white micas are generally One is the phengitic substitution (x), which preserves the described as solid solutions between the muscovite pure dioctahedral character of the mica; the second is the end member K(A12R)(Si3AI)Olo(OH)2, where [] biotitic substitution (y), which leads to trioctahedral stands for an octahedral vacant site, and the micas and does not change the composition of the celadonite end member K(AIM z+ [3)Si4010(OH)2, tetrahedral layer Si3AI. The general formula of muscovite with M 2 + = Mg 2., Fe E+, thus, they are considered in this system is K(A12_~_2y/aM2+yOl_y/a)(Sia+~All_x) to belong to the so-called phengitic series. -

List of Abbreviations
List of Abbreviations Ab albite Cbz chabazite Fa fayalite Acm acmite Cc chalcocite Fac ferroactinolite Act actinolite Ccl chrysocolla Fcp ferrocarpholite Adr andradite Ccn cancrinite Fed ferroedenite Agt aegirine-augite Ccp chalcopyrite Flt fluorite Ak akermanite Cel celadonite Fo forsterite Alm almandine Cen clinoenstatite Fpa ferropargasite Aln allanite Cfs clinoferrosilite Fs ferrosilite ( ortho) Als aluminosilicate Chl chlorite Fst fassite Am amphibole Chn chondrodite Fts ferrotscher- An anorthite Chr chromite makite And andalusite Chu clinohumite Gbs gibbsite Anh anhydrite Cld chloritoid Ged gedrite Ank ankerite Cls celestite Gh gehlenite Anl analcite Cp carpholite Gln glaucophane Ann annite Cpx Ca clinopyroxene Glt glauconite Ant anatase Crd cordierite Gn galena Ap apatite ern carnegieite Gp gypsum Apo apophyllite Crn corundum Gr graphite Apy arsenopyrite Crs cristroballite Grs grossular Arf arfvedsonite Cs coesite Grt garnet Arg aragonite Cst cassiterite Gru grunerite Atg antigorite Ctl chrysotile Gt goethite Ath anthophyllite Cum cummingtonite Hbl hornblende Aug augite Cv covellite He hercynite Ax axinite Czo clinozoisite Hd hedenbergite Bhm boehmite Dg diginite Hem hematite Bn bornite Di diopside Hl halite Brc brucite Dia diamond Hs hastingsite Brk brookite Dol dolomite Hu humite Brl beryl Drv dravite Hul heulandite Brt barite Dsp diaspore Hyn haiiyne Bst bustamite Eck eckermannite Ill illite Bt biotite Ed edenite Ilm ilmenite Cal calcite Elb elbaite Jd jadeite Cam Ca clinoamphi- En enstatite ( ortho) Jh johannsenite bole Ep epidote -
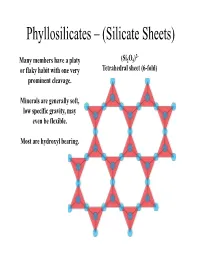
Phyllosilicates – (Silicate Sheets)
Phyllosilicates – (Silicate Sheets) 2- Many members have a platy (Si2O5) or flaky habit with one very Tetrahedral sheet (6-fold) prominent cleavage . Minerals are generally soft, low specific gravity, may even be flexible. Most are hydroxyl bearing. Each tetrahedra is bound to three neiggghboring tetrahedra via three basal bridging oxygens. The apical oxygen of each tetrahedral in a sheet all point in the same direction. The sheets are stacked either apice-to- apice or base-to-base. In an undistorted sheet the hydroxyl (OH) group sits in the centre and each outlined triangle is equivalent. Sheets within sheets…. Apical oxygens, plus the –OH group, coordinate a 6-fold (octahedral) site (XO6). These octahedral sites form infinitely extending sheets. All the octahedra lie on triangular faces, oblique to the tetrahedral sheets. The most common elements found in the 6 -fold site are Mg (or Fe) or Al . Dioctahedral vs Trioctahedral Mg and Al have different charges, but the sheet must remain charge neutral . With 6 coordinating oxygens, we have a partial charge of -6. How many Mg2+ ions are required to retain neutrality? How many Al3+ ions are required to retain neutrality? Mg occupies all octahedral sites, while Al will only occupy 2 out of every 3. The stacking of the sheets dictates the crystallography and c hem istry o f eac h o f t he p hhyll llosili cates. Trioctahedral Dioctahedral O Brucite Gibbsite Hydroxyl Magnesium Aluminium Trioctahedral Is this structure charge neutral? T O T Interlayer Cation T O T Potassium (K+) Phlogopite (Mg end-member biotite) Dioctahedral Is this structure charge neutral? T O T Interlayer Cation T O T Potassium (K+) Muscovite Compositional variation in phyllosilicates There is little solid solution between members of the dioctahedral and trioctahedral groups. -

Stability of Biotite: Experiment
THE AMERICAN MINERALOGIST, VOL. 50. SEPTEMBER, 1965 STABILITY OF BIOTITE: EXPERIMENT. THEORY, AND APPLICATION' Dlvrn R. WoNes, U. S. GeologicalSuraey, Washington, D. C. AND HaNs P. Eucstnn, Deparlment of Geology,The Johns H opkins U niter sity, Baltimore, M arylond'. AesrRecr Biotites on the join phlogopite-annite react to form a number of assemblages and the reactions are governed by the independent intensive parameters, temperature, fugacity of HrO (fnro), and fugacity of oxygen (f6 r) . The most common of these assemblagesin natural occurrences are biotite-sanidine-hematite; biotite-sanidine-magnetite; and biotite-leucite- olivine-magnetite. The compositions of biotites coexisting with sanidine and magnetite were determined for a variety of conditions of furo, fs, and temperature. The compositions lie in the ternary system KFes2+AlSLOro (OH)r, annite-KMg32+AlSraOro (OII)2, phlogo- pite-KFe3r+AlSigOu (H-J, "oxybiotite." Application of regular solution theory to KFerAlSiaOro(OH)s in ternary solid solution yields the following relationship: 3428- 4212(1 - x'), log fsrg : log xr l/2logfo,+ 8.23 - log aKArsi"or- log ap",e T * I where x1 is the mol fraction of KFeaAlSisOro(OH)z in biotite, ar<,rrsi.o,is the activity of KAISLOs in the various mineral phases, and aFg,e,is the activity of FesOr in the various mineral phases. Neither molecular (a: x) or cationic (a: t') ideal solution theory describes the activities of KFedlSLOro(OH)e in "ternary" biotite solid solutions. The position of the fe.-Tcurvesfor biotite-muscovite-magnetite-corundum/alumino-silicate-quartzassemblages were determined by the intersections of the stability curves of biotite and muscovite. -

Nomenclature of the Micas
Mineralogical Magazine, April 1999, Vol. 63(2), pp. 267-279 Nomenclature of the micas M. RIEDER (CHAIRMAN) Department of Geochemistry, Mineralogy and Mineral Resources, Charles University, Albertov 6, 12843 Praha 2, Czech Republic G. CAVAZZINI Dipartimento di Mineralogia e Petrologia, Universith di Padova, Corso Garibaldi, 37, 1-35122 Padova, Italy Yu. S. D'YAKONOV VSEGEI, Srednii pr., 74, 199 026 Sankt-Peterburg, Russia W. m. FRANK-KAMENETSKII* G. GOTTARDIt S. GUGGENHEIM Department of Geological Sciences, University of Illinois at Chicago, 845 West Taylor St., Chicago, IL 60607-7059, USA P. V. KOVAL' Institut geokhimii SO AN Rossii, ul. Favorskogo la, Irkutsk - 33, Russia 664 033 G. MOLLER Institut fiir Mineralogie und Mineralische Rohstoffe, Technische Universit/it Clausthal, Postfach 1253, D-38670 Clausthal-Zellerfeld, Germany A. M, R. NEIVA Departamento de Ci6ncias da Terra, Universidade de Coimbra, Apartado 3014, 3049 Coimbra CODEX, Portugal E. W. RADOSLOVICH$ J.-L. ROBERT Centre de Recherche sur la Synth6se et la Chimie des Min6raux, C.N.R.S., 1A, Rue de la F6rollerie, 45071 Od6ans CEDEX 2, France F. P. SASSI Dipartimento di Mineralogia e Petrologia, Universit~t di Padova, Corso Garibaldi, 37, 1-35122 Padova, Italy H. TAKEDA Chiba Institute of Technology, 2-17-1 Tsudanuma, Narashino City, Chiba 275, Japan Z. WEISS Central Analytical Laboratory, Technical University of Mining and Metallurgy, T/'. 17.1istopadu, 708 33 Ostrava- Poruba, Czech Republic AND D. R. WONESw * Russia; died 1994 t Italy; died 1988 * Australia; resigned 1986 wUSA; died 1984 1999 The Mineralogical Society M. RIEDER ETAL. ABSTRACT I I End-members and species defined with permissible ranges of composition are presented for the true micas, the brittle micas, and the interlayer-deficient micas. -

Petrology and Geochemistry of Selected Talc-Bearing Ultramafic Rocks and Adjacent Country Rocks in North-Central Vermont
Petrology and Geochemistry of Selected Talc-bearing Ultramafic Rocks and Adjacent Country Rocks in North-Central Vermont GEOLOGICAL SURVEY PROFESSIONAL PAPER 345 Petrology and Geochemistry of Selected Talc-bearing Ultramafic Rocks and Adjacent Country Rocks in North-Central Vermont By ALFRED H. CHIDESTER GEOLOGICAL SURVEY PROFESSIONAL PAPER 345 UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1962 UNITED STATES DEPARTMENT OF THE INTERIOR STEW ART L. UDALL, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director The U.S. Geological Survey Library has cataloged this publication as follows : Chidester, Alfred Herman, 1914- Petrology and geochemistry of selected talc bearing- ultra- mafic rocks and adjacent country rocks in north-central Vermont. Washington, U.S. Govt. Print. Off., 1961. vii, 207 p. illus., maps (7 fold. col. in pocket) cliagrs.. tables. 29 cm. (U.S. Geological Survey. Professional paper 345) Bibliography: p. 205-207. 1. Petrology Vermont. 2. Talc Vermont. 3. Mines and mineral resources Vermont. 4. Geochemistry Vermont. 5. Rocks, Igne ous Vermont. I. Title. (Series) For sale by the Superintendent of Documents, U.S. Government Printing Office Washington 25, B.C. CONTENTS Page Geology of the Barnes Hill, Waterbury mine, and Mad Abstract. __________________________________________ 1 River localities Continued Introduction. ______________________________________ 3 Petrography Continued Location and history. ___________________________ 3 Schist Continued Barnes Hill locality _________________________ 3 Mineralogy, etc. Continued Page Waterbury mine locality_____--_---__________ 4 . Ilmenite, rutile, and sphene_ _________ 52 Mad River locality_________---_----__-______ 4 Garnet.___________________________ 52 Previous investigations __________________________ 5 Apatite__. _________________________ 53 Fieldwork and acknowledgments. -___-_---________ 5 Epidote and allanite _______________ 53 Geologic setting. ___________________________________ 6 Other minerals _____________________ 53 Regional setting. -

The Structural Formula of Talc from the Trimouns
997 TheCanatlian Mineralo gist Vo].37, pp. 997-1006 (1999) THESTRUCTURAL FORMULA OF TALCFROM THE TRIMOUNS DEPOSIT, PYRENEES.FRANCE FRANQOIS MARTIN$ ANDPIERRE MICOUD Inboratoire de Mindralogie,Universitd Paul Sabatier-Toulouse III, UMR 5563du CNRS,39, Alldes Jules Guesde, F-31000 Toulouse. France LUC DELMOTTE, CLAIRE MARICHAL ANDRONAN LE DRED LaboratoireMatdrieux Mindraux, URA 0428, CNRS, Ecole Nationale Supdrieure de Chimiede Mulhouse, j, rueAlfred Werner,F-68093 Mulhouse Ceder, France PHILIPPE DE PARSEVAL Laboratoirede Mindralogie,Universitd Paul Sabatier-Toulouse III, UMR 5563du CNRS,39, All4es Jules Guesde, F-31000 Toulouse. France ALAIN MARI ktboratoire de Chimiede Coordination,CNRS,205 Route de Narbonne,F-31077 Toulouse Cedex, France JEAN-POL FORTUNE, STEFANO SALVI ANDDIDIER B6ZIAT Laboratoirede Mindralogie,Universitd Paul Sabatier ToulouseIII, UMR 5563du CNRS,39, All4es Jules Guesde, F-31000 Toulouse, France OLIVIER GRAUBY Centrede Recherche de la Moddlisationet de la CroissanceCristalline, CNRS, Campus de Luminy,Case 913, F-13288Marseille Cedex 9. France JOCELYNEFERRET Talc de Luzenac 5.A., BP 1162, F-31036 Toulouse Ceder, France Assrnacr A sample of talc (Tl 11) from the Trimouns deposit, in the Pyr6n6es,France, was studied by various spectroscopicmethods (FTIR, NMR, EPR and M<issbauer),as well as HRTEM and X-ray diffraction, in order to investigate the extent of minor substi- tutions in the tetrahedraland octahedral sites. Substitutions causea deficiency in tetrahedralcharge, compensated by an excessin octahedral charge, ensuring -

Z-Fessiaoro(OH)R Wennbn C. Fonnos, Unilersity Oj Illinois at Chicagocircl,E, Chicago,Illinois 60680
THE AMERICAN MINERALOGIST. VOL. 54, SEPTEMBER OCTOBER, 1969 UNIT_CELL PARAMETERS AND OPTICAL PROPERTIES OF TALC ON THE JOIN MgaSinOro(OH)z-FesSiaOro(OH)r WennBN C. Fonnos, Unilersity oJ Illinois at ChicagoCircl,e, Chicago,Illinois 60680. Assrnacr Values of d(003), d(}ffi), and ? were measured for talcs of varying Fe/(Fef Mg) ratios synthesized under controlled conditions of temperature, pressure, and oxygen fugacity' The effect of iron on the d(003) spacing is strongly dependent on oxygen fugacity. Iron substitution produces an increase in the c dimension, with the amount of increase greatest for the hematite-magnetite (HM) buffer series and lowest for the magnetite-wtstite, mag- netite-iron series (MW-MI). The b dimension increases regularly with iron content oniy on the MW-MI buffer. At higher relative oxygen fugacities, d(060) may be slightly larger than that of iron-free talc, but does not vary significantly with further iron substitution. The index,7, increases with iron content, but there is no apparent correlation between 7 and oxygen fugacity. A substitution of the type Fe3++H+:Si4+ is proposed to explain the observed varia- tions. The increase in c may result from the formation of hydroxyl ions on the basal sur- face. The extent of this substitution is apparently greatest on the HM bufier and decreases at lower oxl'gen fugacities. The pronounced effect of oxygen fugacity on the cell dimensions of iron-bearing talc suggest that talc may be of some use as an indicator of oxygen fugacity in natural environ- ments, INrnonuctror.r The effect of iron substitution on the unit cell parameters and optical properties of talc was determined as part of a study of the phaseequilibria of the system MgaSiaOro(OH)2-Fe3SiaOlo(OH)2.These data were required for determining the iron content of fine grained talc coexisting with other iron-bearingphases. -

Phyllosilicates (Micas, Chlorite, Talc, & Serpentine)
Phyllosilicates EENS 211 Mineralogy Tulane University Prof. Stephen A. Nelson Phyllosilicates (Micas, Chlorite, Talc, & Serpentine) This document last updated on 12-Nov-2008 Phyllosilicates (Sheet Silicates) The phyllosilicates, or sheet silicates, are an important group of minerals that includes the micas, chlorite, serpentine, talc, and the clay minerals. Because of the special importance of the clay minerals as one of the primary products of chemical weathering and one of the more abundant constituents of sedimentary rocks, they will be discussed in more detail in the next lecture. The basic structure of the phyllosilicates is based on -4 interconnected six member rings of SiO4 tetrahedra that extend outward in infinite sheets. Three out of the 4 oxygens from each tetrahedra are shared with other -2 tetrahedra. This leads to a basic structural unit of Si2O5 . Most phyllosilicates contain hydroxyl ion, OH-, with the OH located at the center of the 6 membered rings, as shown here. -3 Thus, the group becomes Si2O5(OH) . When other cations are bonded to the SiO4 sheets, they share the apical oxygens and the (OH) ions which bond to the other cations in octahedral coordination. This forms a layer of cations, usually Fe+2, Mg+2, or Al+3, that occur in octahedral coordination with the O and OH ions of the tetrahedral layer. As shown, here, the triangles become the faces of the octahedral groups that can bind to the tetrahedral layers. The octahedral layers take on the structure of either Brucite [Mg(OH)3], if the cations are +2 ions like Mg+2 or Fe+2, or Gibbsite +3 [Al(OH)3], if the cations are +3 like Al . -

Crystal Chemistry of Trioctahedral Micas-2M1 from Bunyaruguru Kamafugite (Southwest Uganda)
American Mineralogist, Volume 97, pages 430–439, 2012 Crystal chemistry of trioctahedral micas-2M1 from Bunyaruguru kamafugite (southwest Uganda) FERNANDO SCORDARI,* EMANUELA SCHINGARO, MARIA LACALAMITA, AND ERNESTO MESTO Dipartimento di Scienze della Terra e Geoambientali, Università degli Studi di Bari, via E. Orabona 4, I-70125 Bari, Italy ABSTRACT The crystal chemistry of 2M1 micas from Bunyaruguru kamafugite (southwest Uganda) was studied by electron probe microanalysis, single-crystal X-ray diffraction, Mössbauer and Fourier transform infrared spectroscopy. Chemical analyses showed that the studied crystals are Ti-rich, F-poor phlogo- pites with an annitic component, Fetot/(Fetot + Mg), ranging from 0.15 to 0.22. Unit-cell parameters from single-crystal X-ray data are in the range: 5.3252(1) ≤ a ≤ 5.3307(1), 9.2231(3) ≤ b ≤ 9.2315(3), 20.1550(6) ≤ c ≤ 20.1964(8) Å, and 94.994(2) ≤ β ≤ 95.131(2)°. Anisotropic structure refinements, in the space group C2/c, converged to 2.77 ≤ R1 ≤ 3.52% and VI 2+ 2.91 ≤ wR2 ≤ 4.02%. Mössbauer spectroscopy showed that the studied sample has: Fe = 60(1)%, VIFe3+ = 24(1)%, and IVFe3+ = 16(1)%. FTIR investigations pointed to the occurrence of Fe3+-oxy sub- stitutions and ruled out the presence of vacancy mechanisms. The overall crystal-chemical features are consistent with the following substitutions: tetraferriphlogopite [IVFe3+ ↔ IVAl]; Ti-oxy [VIM2+ + − VI 4+ 2– 3+ VI 2+ − VI 3+ 2– 2 (OH) ↔ Ti + 2 (O ) + H2↑] and Al, Fe , Cr-oxy [ M + (OH) ↔ M + O + ½ (H2)↑]; Al, Fe3+-Tschermak [VIM2+ + IVSi4+ ↔ VIM3+ + IVAl]; kinoshitalite [XIIK + IVSi4+ ↔ XIIBa2+ + IVAl] and [XIIK+ + IVAl3+ ↔ IVSi4+ + XII]. -

Mica-Vermiculite Intergrowth Expansion Through Natural Processes in Pyroclastic Carbonatites Fromcatanda (Angola)
Scientiic Research Abstracts Vol. 7, p. 808, 2017 ISSN 2464-9147 (Online) XVI International Clay Conference | ICC 2017 | Granada, Spain © Author(s) 2017. CC Attribution 3.0 License MICA-VERMICULITE INTERGROWTH EXPANSION THROUGH NATURAL PROCESSES IN PYROCLASTIC CARBONATITES FROMCATANDA (ANGOLA) jinGyao xu (1)*, marC Campeny (1), esperança tauler (1), joan Carles melGarejo (1), antonio olimpio Gonçalves (2) (1) Departament de Mineralogia, Petrologia i Geologia Aplicada, Facultat de Geologia, Universitat de Barcelona, 08028 Barcelona, Spain, (2) Departamento de Geologia, Universidade Agostinho Neto, Luanda, Angola *[email protected] Catanda carbonatites are found in the Kwanza Sul province, about 350 km SE of Luanda (Angola). Theseare formed by small volcanic cones consisting of a series of pyroclastic rocks with minor interbedded carbonatitic lavas [1]. Phlogopite as a carbonatitic mineral and annite as xenocrystal provided from hosted granites are found in the Catanda pyroclastic carbonatites. Secondary minerals such as vermiculite have also been reported [1]. Under petrographic study, phlogopite and annite present textures as non-expanded, slightly expanded and strongly expanded deining accordion texture. Vermiculite can be distinguished using SEM-EDS in back-scattered electron (BSE) mode. Bothphlogopite and annite are affected by the vermiculitization process. Previous work [2,3] has proposed that vermiculitization of micas proceeds through the sequence: mica to inter- stratiied mica-vermiculite to vermiculite, and this is determined byXRD as peaks at 10, 12 and14 Å, respectively. In the present study of the Catanda samples, diffraction peaks were identiied at 10 and 14 Å only, and no 12 Åpeak was found. We propose that the Catanda intermediate product of phlogopite-vermiculiteshould not be considered as an interstratiied phlogopite-vermiculite but a mixture of phlogopite and vermiculite, consisting of intergrowths of both minerals inan intermediate stage of alteration, similar to theintergrowth of phyllosilicates described inother works [4,5].