Muscovite Solid Solutions in the System K20-Mgo-Feo-A1203-Sio2-H20: an Experimental Study at 2 Kbar Ph2o and Comparison with Natural Li-Free White Micas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download PDF About Minerals Sorted by Mineral Name
MINERALS SORTED BY NAME Here is an alphabetical list of minerals discussed on this site. More information on and photographs of these minerals in Kentucky is available in the book “Rocks and Minerals of Kentucky” (Anderson, 1994). APATITE Crystal system: hexagonal. Fracture: conchoidal. Color: red, brown, white. Hardness: 5.0. Luster: opaque or semitransparent. Specific gravity: 3.1. Apatite, also called cellophane, occurs in peridotites in eastern and western Kentucky. A microcrystalline variety of collophane found in northern Woodford County is dark reddish brown, porous, and occurs in phosphatic beds, lenses, and nodules in the Tanglewood Member of the Lexington Limestone. Some fossils in the Tanglewood Member are coated with phosphate. Beds are generally very thin, but occasionally several feet thick. The Woodford County phosphate beds were mined during the early 1900s near Wallace, Ky. BARITE Crystal system: orthorhombic. Cleavage: often in groups of platy or tabular crystals. Color: usually white, but may be light shades of blue, brown, yellow, or red. Hardness: 3.0 to 3.5. Streak: white. Luster: vitreous to pearly. Specific gravity: 4.5. Tenacity: brittle. Uses: in heavy muds in oil-well drilling, to increase brilliance in the glass-making industry, as filler for paper, cosmetics, textiles, linoleum, rubber goods, paints. Barite generally occurs in a white massive variety (often appearing earthy when weathered), although some clear to bluish, bladed barite crystals have been observed in several vein deposits in central Kentucky, and commonly occurs as a solid solution series with celestite where barium and strontium can substitute for each other. Various nodular zones have been observed in Silurian–Devonian rocks in east-central Kentucky. -

Chemical and Structural Evolution of “Metamorphic Vermiculite” in Metaclastic Rocks of the Betic Cordillera, Málaga, Spain: a Synthesis
249 The Canadian Mineralogist Vol. 44, pp. 249-265 (2006) CHEMICAL AND STRUCTURAL EVOLUTION OF “METAMORPHIC VERMICULITE” IN METACLASTIC ROCKS OF THE BETIC CORDILLERA, MÁLAGA, SPAIN: A SYNTHESIS MARÍA DOLORES RUIZ CRUZ§ Departamento de Química Inorgánica, Cristalografía y Mineralogía, Universidad de Málaga, Campus de Teatinos, E-29071 Málaga, Spain JOSÉ MIGUEL NIETO Departamento de Geología, Facultad de Ciencias Experimentales, Universidad de Huelva, E-21071 Huelva, Spain ABSTRACT Vermiculite-like minerals from a metamorphic sequence of the Betic Cordillera, near Málaga, southeastern Spain, were investigated in detail by X-ray diffraction, electron-microprobe analysis, and transmission-analytical electron microscopy. Our results reveal that the chlorite-to-biotite transformation is much more complex than previously assumed. In addition to mixed- layer minerals with a mica:chlorite ratio of 2:1 and 1:1, which had previously been identifi ed, mixed-layer phases with very high and very low chlorite:vermiculite ratios have been identifi ed, together with true vermiculite, as intermediate steps in the chlorite-to-biotite transformation. The observed sequence is: chlorite → random to ordered 1:2 mica–chlorite mixed-layer phases → regular 1:1 chlorite–vermiculite mixed-layer phases → Vrm-rich chlorite–vermiculite mixed-layer phases → vermiculite → biotite. This sequence includes a continuous increase of interlayer cation content, and is similar to that described in the smectite- to-illite transformation. The high Na content of most of these phases suggests that in the absence of K-feldspar, two parallel sites of reactants, chlorite + phengite and chlorite + albite, account for the formation of vermiculitic phases, and later, of biotite. Keywords: mixed-layer minerals, metamorphic vermiculite, X-ray diffraction, electron-microprobe analysis, transmission elec- tron microscopy, analytical electron microscopy, Betic Cordillera, Spain. -

Clay Minerals Soils to Engineering Technology to Cat Litter
Clay Minerals Soils to Engineering Technology to Cat Litter USC Mineralogy Geol 215a (Anderson) Clay Minerals Clay minerals likely are the most utilized minerals … not just as the soils that grow plants for foods and garment, but a great range of applications, including oil absorbants, iron casting, animal feeds, pottery, china, pharmaceuticals, drilling fluids, waste water treatment, food preparation, paint, and … yes, cat litter! Bentonite workings, WY Clay Minerals There are three main groups of clay minerals: Kaolinite - also includes dickite and nacrite; formed by the decomposition of orthoclase feldspar (e.g. in granite); kaolin is the principal constituent in china clay. Illite - also includes glauconite (a green clay sand) and are the commonest clay minerals; formed by the decomposition of some micas and feldspars; predominant in marine clays and shales. Smectites or montmorillonites - also includes bentonite and vermiculite; formed by the alteration of mafic igneous rocks rich in Ca and Mg; weak linkage by cations (e.g. Na+, Ca++) results in high swelling/shrinking potential Clay Minerals are Phyllosilicates All have layers of Si tetrahedra SEM view of clay and layers of Al, Fe, Mg octahedra, similar to gibbsite or brucite Clay Minerals The kaolinite clays are 1:1 phyllosilicates The montmorillonite and illite clays are 2:1 phyllosilicates 1:1 and 2:1 Clay Minerals Marine Clays Clays mostly form on land but are often transported to the oceans, covering vast regions. Kaolinite Al2Si2O5(OH)2 Kaolinite clays have long been used in the ceramic industry, especially in fine porcelains, because they can be easily molded, have a fine texture, and are white when fired. -

Part 629 – Glossary of Landform and Geologic Terms
Title 430 – National Soil Survey Handbook Part 629 – Glossary of Landform and Geologic Terms Subpart A – General Information 629.0 Definition and Purpose This glossary provides the NCSS soil survey program, soil scientists, and natural resource specialists with landform, geologic, and related terms and their definitions to— (1) Improve soil landscape description with a standard, single source landform and geologic glossary. (2) Enhance geomorphic content and clarity of soil map unit descriptions by use of accurate, defined terms. (3) Establish consistent geomorphic term usage in soil science and the National Cooperative Soil Survey (NCSS). (4) Provide standard geomorphic definitions for databases and soil survey technical publications. (5) Train soil scientists and related professionals in soils as landscape and geomorphic entities. 629.1 Responsibilities This glossary serves as the official NCSS reference for landform, geologic, and related terms. The staff of the National Soil Survey Center, located in Lincoln, NE, is responsible for maintaining and updating this glossary. Soil Science Division staff and NCSS participants are encouraged to propose additions and changes to the glossary for use in pedon descriptions, soil map unit descriptions, and soil survey publications. The Glossary of Geology (GG, 2005) serves as a major source for many glossary terms. The American Geologic Institute (AGI) granted the USDA Natural Resources Conservation Service (formerly the Soil Conservation Service) permission (in letters dated September 11, 1985, and September 22, 1993) to use existing definitions. Sources of, and modifications to, original definitions are explained immediately below. 629.2 Definitions A. Reference Codes Sources from which definitions were taken, whole or in part, are identified by a code (e.g., GG) following each definition. -

06 11 09 Separation & Preparation of Biotite and Muscovite Samples For
06 11 09 Separation & Preparation of Biotite and Muscovite samples for 40Ar/39Ar analysis Karl Lang When dealing with detrital samples, sample contamination is a big problem. To avoid this at all costs be clean, this means handling samples one at a time and cleaning all equipment entirely after each sample handling. Only handle samples in a quite (i.e. not windy) environment, where there is little chance of spilling or blowing samples away. Handle samples over clean copier paper, and change the paper after each sample. Use compressed air (either canned or from a compressor) to clean all equipment in separation stages and methanol to keep equipment dirt and dust free in the preparation stages. This can be often be tedious work, I recommend finding a good book on tape or podcast to listen to. Estimate times to completion are stated. Detrital Samples Separation I. Sample drying (1-3 days) For detrital samples it is likely that samples will be wet. Simple let the samples dry in the open air, or under a mild lamp on paper plates. It may be necessary to occasionally stir up samples to get them to dry faster, if you do this, clean the stirrer after each sample. Split samples using a riffle splitter to obtain a quantity for the rest of the process, do this in the rock room. II. Sample Sieving (3-7 days) First locate a set of sieves that can be intensively cleaned. There are a set of appropriate sieves in 317 for this use, be careful using other's sieves as you will likely bend the meshes during cleaning. -
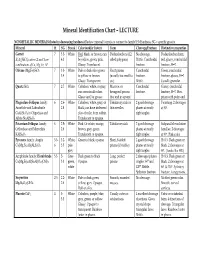
Mineral Identification Chart – LECTURE
Mineral Identification Chart – LECTURE NONMETALLIC MINERALS (listed in decreasing hardness) Review mineral formula to connect to family! H=Hardness; SG = specific gravity Mineral H SG Streak Color (and/or luster) Form Cleavage/Fracture Distinctive properties Garnet 7 3.5- White Red, black, or brown; can Dodecahedrons (12- No cleavage. Dodecahedron form, X3Y2(SiO4)3 where X and Y are 4.3 be yellow, green, pink. sided polygons) Brittle. Conchoidal red, glassy, conchoidal combinations of Ca, Mg, Fe, Al Glassy. Translucent. fracture. fracture, H=7. Olivine (Mg,Fe)2SiO4 7 3.3- White Pale or dark olive green Short prisms Conchoidal Green, conchoidal 3.4 to yellow or brown. (usually too small to fracture. fracture, glassy, H=7. Glassy. Transparent. see). Brittle. Usually granular. Quartz SiO2 7 2.7 White Colorless, white, or gray; Massive; or Conchoidal Glassy, conchoidal can occur in all colors. hexagonal prisms fracture. fracture, H=7. Hex. Glassy and/or greasy. that end in a point. prism with point end. Plagioclase Feldspar family: 6 2.6- White Colorless, white, gray, or Tabular crystals or 2 good cleavage Twinning. 2 cleavages Anorthite and Labradorite 2.8 black; can have iridescent thin needles planes at nearly at 90°. CaAl2Si2O8 to Oligoclase and play of color from within. right angles. Albite NaAlSi3O8 Translucent to opaque. Potassium Feldspar family: 6 2.5- White Pink. Or white, orange, Tabular crystals 2 good cleavage Subparallel exsolution Orthoclase and Microcline 2.6 brown, gray, green. planes at nearly lamellae. 2 cleavages KAlSi3O8 Translucent to opaque. right angles. at 90°. Pink color. Pyroxene family: Augite 5.5- 3.2- White, Green to black; opaque. -

Cr3+ in Phyllosilicates
Mineral Spectroscopy: A Tribute to Roger G. Bums © The Geochemical Society, Special Publication No.5, ]996 Editors: M. D. Dyar, C. McCammon and M. W. Schaefer 3 Cr + in phyllosilicates: Influence of the nature of coordinating ligands and their next cationic neighbors on the crystal field parameters I 2 2 A. N. PLATONOV , K. LANGER , M. ANDRUT .3, G. CALAS4 'Institute of Geochemistry, Mineralogy and Ore Formation, Academy of Science of Ukraine, 252680 Kiev, Ukraine 2Institute of Mineralogy and Crystallography, Technical University, D-10623 Berlin, Germany 3GeoForschungszentrum Potsdam, D-14473 Potsdam, Deutschland "Laboratoire de Mineralogie et de Cristallographie, Universite de Paris 6 et 7, F-7525l Paris, France 3 Abstract- The electronic absorption spectra of Cr + -bearing clinochlore (I, kammererite), amesite (II), muscovite (III, fuchsite), dickite (IV), and montmorillonite (V, volkonskite) analysed by electron microprobe were obtained on single crystals. Microscope-spectrometric techniques and polarized radiation in the spectral range 10000-38000 cm " (I, II, III) or (on fine grained material) diffuse reflectance spectrometry in the spectral range 8000-50000 cm-I (IV, V) were used. The ligand field theoretical evaluation of the spectra showed the following: (i) The fl.o = 10Dq = f(1/R5) relation, wherein fl.o is the octahedral crystal field parameter and R the mean cation ligand distance, is valid within each series of layer silicates containing octahedral Cr3+ either in a trioctahedral layer (I, II and phlogopite) or in a dioctahedral layer (III, IV, V). Between the two functions, fl.o.trioct = f(1lR~ioct) and fl.o.di=t = f(1/R~ioct), there exists an energy difference of about 2200 em -I. -

Phyllosilicates in the Sediment-Forming Processes: Weathering, Erosion, Transportation, and Deposition
Acta Geodyn. Geomater., Vol. 6, No. 1 (153), 13–43, 2009 PHYLLOSILICATES IN THE SEDIMENT-FORMING PROCESSES: WEATHERING, EROSION, TRANSPORTATION, AND DEPOSITION Jiří KONTA Faculty of Sciences, Charles University, Albertov 6, 128 43 Prague 2 Home address: Korunní 127, 130 00 Prague 3, Czech Republic *Corresponding author‘s e-mail: [email protected] (Received October 2008, accepted January 2009) ABSTRACT Phyllosilicates are classified into the following groups: 1 - Neutral 1:1 structures: the kaolinite and serpentine group. 2 - Neutral 2:1 structures: the pyrophyllite and talc group. 3 - High-charge 2:1 structures, non-expansible in polar liquids: illite and the dioctahedral and trioctahedral micas, also brittle micas. 4 - Low- to medium-charge 2:1 structures, expansible phyllosilicates in polar liquids: smectites and vermiculites. 5 - Neutral 2:1:1 structures: chlorites. 6 - Neutral to weak-charge ribbon structures, so-called pseudophyllosilicates or hormites: palygorskite and sepiolite (fibrous crystalline clay minerals). 7 - Amorphous clay minerals. Order-disorder states, polymorphism, polytypism, and interstratifications of phyllosilicates are influenced by several factors: 1) a chemical micromilieu acting during the crystallization in any environment, including the space of clay pseudomorphs after original rock-forming silicates or volcanic glasses; 2) the accepted thermal energy; 3) the permeability. The composition and properties of parent rocks and minerals in the weathering crusts, the elevation, and topography of source areas and climatic conditions control the intensity of weathering, erosion, and the resulting assemblage of phyllosilicates to be transported after erosion. The enormously high accumulation of phyllosilicates in the sedimentary lithosphere is primarily conditioned by their high up to extremely high chemical stability in water-rich environments (expressed by index of corrosion, IKO). -

List of Abbreviations
List of Abbreviations Ab albite Cbz chabazite Fa fayalite Acm acmite Cc chalcocite Fac ferroactinolite Act actinolite Ccl chrysocolla Fcp ferrocarpholite Adr andradite Ccn cancrinite Fed ferroedenite Agt aegirine-augite Ccp chalcopyrite Flt fluorite Ak akermanite Cel celadonite Fo forsterite Alm almandine Cen clinoenstatite Fpa ferropargasite Aln allanite Cfs clinoferrosilite Fs ferrosilite ( ortho) Als aluminosilicate Chl chlorite Fst fassite Am amphibole Chn chondrodite Fts ferrotscher- An anorthite Chr chromite makite And andalusite Chu clinohumite Gbs gibbsite Anh anhydrite Cld chloritoid Ged gedrite Ank ankerite Cls celestite Gh gehlenite Anl analcite Cp carpholite Gln glaucophane Ann annite Cpx Ca clinopyroxene Glt glauconite Ant anatase Crd cordierite Gn galena Ap apatite ern carnegieite Gp gypsum Apo apophyllite Crn corundum Gr graphite Apy arsenopyrite Crs cristroballite Grs grossular Arf arfvedsonite Cs coesite Grt garnet Arg aragonite Cst cassiterite Gru grunerite Atg antigorite Ctl chrysotile Gt goethite Ath anthophyllite Cum cummingtonite Hbl hornblende Aug augite Cv covellite He hercynite Ax axinite Czo clinozoisite Hd hedenbergite Bhm boehmite Dg diginite Hem hematite Bn bornite Di diopside Hl halite Brc brucite Dia diamond Hs hastingsite Brk brookite Dol dolomite Hu humite Brl beryl Drv dravite Hul heulandite Brt barite Dsp diaspore Hyn haiiyne Bst bustamite Eck eckermannite Ill illite Bt biotite Ed edenite Ilm ilmenite Cal calcite Elb elbaite Jd jadeite Cam Ca clinoamphi- En enstatite ( ortho) Jh johannsenite bole Ep epidote -

Rock and Mineral Identification for Engineers
Rock and Mineral Identification for Engineers November 1991 r~ u.s. Department of Transportation Federal Highway Administration acid bottle 8 granite ~~_k_nife _) v / muscovite 8 magnify~in_g . lens~ 0 09<2) Some common rocks, minerals, and identification aids (see text). Rock And Mineral Identification for Engineers TABLE OF CONTENTS Introduction ................................................................................ 1 Minerals ...................................................................................... 2 Rocks ........................................................................................... 6 Mineral Identification Procedure ............................................ 8 Rock Identification Procedure ............................................... 22 Engineering Properties of Rock Types ................................. 42 Summary ................................................................................... 49 Appendix: References ............................................................. 50 FIGURES 1. Moh's Hardness Scale ......................................................... 10 2. The Mineral Chert ............................................................... 16 3. The Mineral Quartz ............................................................. 16 4. The Mineral Plagioclase ...................................................... 17 5. The Minerals Orthoclase ..................................................... 17 6. The Mineral Hornblende ................................................... -

Minerals Found in Michigan Listed by County
Michigan Minerals Listed by Mineral Name Based on MI DEQ GSD Bulletin 6 “Mineralogy of Michigan” Actinolite, Dickinson, Gogebic, Gratiot, and Anthonyite, Houghton County Marquette counties Anthophyllite, Dickinson, and Marquette counties Aegirinaugite, Marquette County Antigorite, Dickinson, and Marquette counties Aegirine, Marquette County Apatite, Baraga, Dickinson, Houghton, Iron, Albite, Dickinson, Gratiot, Houghton, Keweenaw, Kalkaska, Keweenaw, Marquette, and Monroe and Marquette counties counties Algodonite, Baraga, Houghton, Keweenaw, and Aphrosiderite, Gogebic, Iron, and Marquette Ontonagon counties counties Allanite, Gogebic, Iron, and Marquette counties Apophyllite, Houghton, and Keweenaw counties Almandite, Dickinson, Keweenaw, and Marquette Aragonite, Gogebic, Iron, Jackson, Marquette, and counties Monroe counties Alunite, Iron County Arsenopyrite, Marquette, and Menominee counties Analcite, Houghton, Keweenaw, and Ontonagon counties Atacamite, Houghton, Keweenaw, and Ontonagon counties Anatase, Gratiot, Houghton, Keweenaw, Marquette, and Ontonagon counties Augite, Dickinson, Genesee, Gratiot, Houghton, Iron, Keweenaw, Marquette, and Ontonagon counties Andalusite, Iron, and Marquette counties Awarurite, Marquette County Andesine, Keweenaw County Axinite, Gogebic, and Marquette counties Andradite, Dickinson County Azurite, Dickinson, Keweenaw, Marquette, and Anglesite, Marquette County Ontonagon counties Anhydrite, Bay, Berrien, Gratiot, Houghton, Babingtonite, Keweenaw County Isabella, Kalamazoo, Kent, Keweenaw, Macomb, Manistee, -

Geology Tour Glossary
GEOLOGY TOUR GLOSSARY ABRASION - a form of mechanical weathering involving the scraping of a rock surface by friction between rocks and moving particles during their transport by wind, glaciers, waves, gravity, running water, or erosion BIOLOGICAL WEATHERING – a type of chemical weathering in which biologically produced chemicals breakdown rocks, soils and minerals BIOTITE - a common dark-brown, dark-green, or black mineral of the mica group CHEMICAL WEATHERING - the direct effect of atmospheric and/or biological chemicals on the breakdown of rocks, soils and minerals COUNTRY ROCK - rock that is native to an area EXFOLIATION - the process in which rocks weather by peeling off in sheets rather that eroding grain by grain FALL ZONE - the geomorphologic break between an upland region of relatively hard crystalline basement rock and a coastal plain of softer sedimentary rock; distinguished by a drop in elevation and waterfalls in rivers FAULT - a planar fracture or discontinuity in a volume of rock, across which there has been significant displacement along the fractures as a result of earth movement FELDSPAR - an abundant, rock-forming mineral that varies in color from pink, yellow-orange, tan-white. Large bits often have squared edges. About 60 percent of the Earth's outer crust is composed of feldspar GEOLOGY - the study of the history and structure of the Earth, the rocks that the Earth is made of, and the processes that form and change the rocks GNEISSIC BANDING - a type of foliation in metamorphic rock consisting of roughly parallel dark and light bands of rock GRANITE - a hard, granular, igneous rock, formed as magma solidifies far below the earth’s surface.